MIR17HG-miR-18a/19a axis, regulated by interferon regulatory factor-1, promotes gastric cancer metastasis via Wnt/β-catenin signalling
- PMID: 31186404
- PMCID: PMC6560107
- DOI: 10.1038/s41419-019-1685-z
MIR17HG-miR-18a/19a axis, regulated by interferon regulatory factor-1, promotes gastric cancer metastasis via Wnt/β-catenin signalling
Abstract
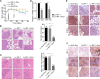
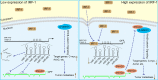
MIR17HG, located on chromosome 13, is a class of Pri-miRNAs that generates six miRNAs: miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92-1. These miRNAs are ubiquitously overexpressed in diverse tumour types and exhibit complex biological links to tumour metastasis. We demonstrated that MIR17HG-derived miR-18a and miR-19a coordinately mediate gastric cancer cell metastasis by directly inhibiting SMAD2 expression and upregulating Wnt/β-catenin signalling. Based on previous studies, we hypothesised that an investigation of MIR17HG inhibition would be beneficial to clinical gastric cancer treatment, and systematically coupled bioinformatics analyses brought interferon regulatory factor-1 (IRF-1) to our attention. We then established stable clones in gastric cancer cells containing a doxycycline-inducible IRF-1 expression system and found that the expression of IRF-1 downregulates the embedded miRNAs of MIR17HG in gastric cancer cells and inhibits gastric cancer cell metastasis by attenuating Wnt/β-catenin signalling. Further rescue assays confirmed the crucial roles of miR-18a and miR-19a in the IRF-1-mediated inhibition of Wnt/β-catenin signalling. We also demonstrated that IRF-1 binds to the transcriptional site in the MIR17HG promoter and inhibits MIR17HG expression. Moreover, IFN-γ induced the IRF-1-mediated downregulation of MIR17HG in gastric cancer cells. Our hypothesis was supported by the results of immunohistochemistry analyses of clinical gastric cancer samples, and we also demonstrated the role of IRF-1 in inhibiting MIR17HG expression and tumour metastasis in vivo. We conclude that IRF-1 inhibits gastric cancer metastasis by downregulating MIR17HG-miR-18a/miR-19a axis expression and attenuating Wnt/β-catenin signalling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Interferon regulatory factor-1 reverses chemoresistance by downregulating the expression of P-glycoprotein in gastric cancer.Cancer Lett. 2019 Aug 10;457:28-39. doi: 10.1016/j.canlet.2019.05.006. Epub 2019 May 9. Cancer Lett. 2019. PMID: 31078735
-
miR-324-3p promotes gastric cancer development by activating Smad4-mediated Wnt/beta-catenin signaling pathway.J Gastroenterol. 2018 Jun;53(6):725-739. doi: 10.1007/s00535-017-1408-0. Epub 2017 Nov 4. J Gastroenterol. 2018. PMID: 29103082 Free PMC article.
-
Aberrantly expressed miR-188-5p promotes gastric cancer metastasis by activating Wnt/β-catenin signaling.BMC Cancer. 2019 May 28;19(1):505. doi: 10.1186/s12885-019-5731-0. BMC Cancer. 2019. PMID: 31138169 Free PMC article.
-
Good or not good: Role of miR-18a in cancer biology.Rep Pract Oncol Radiother. 2020 Sep-Oct;25(5):808-819. doi: 10.1016/j.rpor.2020.07.006. Epub 2020 Aug 12. Rep Pract Oncol Radiother. 2020. PMID: 32884453 Free PMC article. Review.
-
The dual roles of circRNAs in Wnt/β-Catenin signaling and cancer progression.Pathol Res Pract. 2024 Mar;255:155132. doi: 10.1016/j.prp.2024.155132. Epub 2024 Feb 1. Pathol Res Pract. 2024. PMID: 38335783 Review.
Cited by
-
Automatic Text-Mining Approach to Identify Molecular Target Candidates Associated with Metabolic Processes for Myotonic Dystrophy Type 1.Int J Environ Res Public Health. 2023 Jan 27;20(3):2283. doi: 10.3390/ijerph20032283. Int J Environ Res Public Health. 2023. PMID: 36767649 Free PMC article.
-
Synergistic Potential of Antibiotics with Cancer Treatments.Cancers (Basel). 2024 Dec 28;17(1):59. doi: 10.3390/cancers17010059. Cancers (Basel). 2024. PMID: 39796688 Free PMC article. Review.
-
lncRNA PART1 and MIR17HG as ΔNp63α direct targets regulate tumor progression of cervical squamous cell carcinoma.Cancer Sci. 2020 Nov;111(11):4129-4141. doi: 10.1111/cas.14649. Epub 2020 Sep 29. Cancer Sci. 2020. PMID: 32920922 Free PMC article.
-
Interferon Regulatory Factor Family Genes: At the Crossroads between Immunity and Head and Neck Squamous Carcinoma.Dis Markers. 2022 May 26;2022:2561673. doi: 10.1155/2022/2561673. eCollection 2022. Dis Markers. 2022. PMID: 35664436 Free PMC article.
-
MiR-18a Inhibits PI3K/AKT Signaling Pathway to Regulate PDGF BB-Induced Airway Smooth Muscle Cell Proliferation and Phenotypic Transformation.Physiol Res. 2021 Dec 30;70(6):883-892. doi: 10.33549/physiolres.934753. Epub 2021 Oct 30. Physiol Res. 2021. PMID: 34717064 Free PMC article.
References
-
- Gu H, et al. The prognostic efficacy and improvements of the7th edition Union for International Cancer Control tumor-node-metastasis classifications for Chinese patients with gastric cancer: results based on a retrospective three-decade population study. Tumour Biol. 2017;39:1010428317694548. doi: 10.1177/1010428317694548. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

