Fast and selective fluoride ion conduction in sub-1-nanometer metal-organic framework channels
- PMID: 31186413
- PMCID: PMC6560108
- DOI: 10.1038/s41467-019-10420-9
Fast and selective fluoride ion conduction in sub-1-nanometer metal-organic framework channels
Abstract
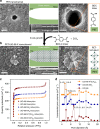
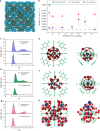
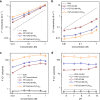
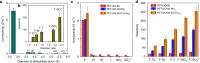
Biological fluoride ion channels are sub-1-nanometer protein pores with ultrahigh F- conductivity and selectivity over other halogen ions. Developing synthetic F- channels with biological-level selectivity is highly desirable for ion separations such as water defluoridation, but it remains a great challenge. Here we report synthetic F- channels fabricated from zirconium-based metal-organic frameworks (MOFs), UiO-66-X (X = H, NH2, and N+(CH3)3). These MOFs are comprised of nanometer-sized cavities connected by sub-1-nanometer-sized windows and have specific F- binding sites along the channels, sharing some features of biological F- channels. UiO-66-X channels consistently show ultrahigh F- conductivity up to ~10 S m-1, and ultrahigh F-/Cl- selectivity, from ~13 to ~240. Molecular dynamics simulations reveal that the ultrahigh F- conductivity and selectivity can be ascribed mainly to the high F- concentration in the UiO-66 channels, arising from specific interactions between F- ions and F- binding sites in the MOF channels.
Conflict of interest statement
H.Z., H.W., X.L., J.L., B.D.F. and A.J.H. are inventors on an international patent application related to this work filed by Monash University (Application No: PCT/AU2018/051341, filed 14 Dec 2018). The authors declare no competing interest.
Figures


References
-
- Ozsvath DL. Fluoride and environmental health: a review. Rev. Environ. Sci. Biotechnol. 2008;8:59–79. doi: 10.1007/s11157-008-9136-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

