Bone mineral: new insights into its chemical composition
- PMID: 31186433
- PMCID: PMC6560110
- DOI: 10.1038/s41598-019-44620-6
Bone mineral: new insights into its chemical composition
Abstract
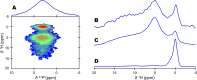
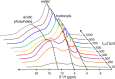
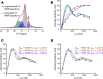
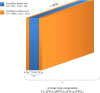
Some compositional and structural features of mature bone mineral particles remain unclear. They have been described as calcium-deficient and hydroxyl-deficient carbonated hydroxyapatite particles in which a fraction of the PO43- lattice sites are occupied by HPO42- ions. The time has come to revise this description since it has now been proven that the surface of mature bone mineral particles is not in the form of hydroxyapatite but rather in the form of hydrated amorphous calcium phosphate. Using a combination of dedicated solid-state nuclear magnetic resonance techniques, the hydrogen-bearing species present in bone mineral and especially the HPO42- ions were closely scrutinized. We show that these HPO42- ions are concentrated at the surface of bone mineral particles in the so-called amorphous surface layer whose thickness was estimated here to be about 0.8 nm for a 4-nm thick particle. We also show that their molar proportion is much higher than previously estimated since they stand for about half of the overall amount of inorganic phosphate ions that compose bone mineral. As such, the mineral-mineral and mineral-biomolecule interfaces in bone tissue must be driven by metastable hydrated amorphous environments rich in HPO42- ions rather than by stable crystalline environments of hydroxyapatite structure.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Glimcher MJ. Bone: Nature of the Calcium Phosphate Crystals and Cellular, Structural, and Physical Chemical Mechanisms in Their Formation. Rev. Mineral. Geochem. 2006;64:223–282. doi: 10.2138/rmg.2006.64.8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

