Dynamic biochemical tissue analysis detects functional selectin ligands on human cancer tissues
- PMID: 31186472
- PMCID: PMC6560120
- DOI: 10.1038/s41598-019-44838-4
Dynamic biochemical tissue analysis detects functional selectin ligands on human cancer tissues
Abstract
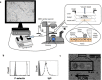
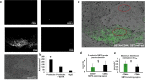
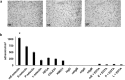
Cell adhesion mediated by selectins (expressed by activated endothelium, activated platelets, and leukocytes) binding to their resepective selectin ligands (expressed by cancer cells) may be involved in metastasis. Therefore, methods of characterizing selectin ligands expressed on human tissue may serve as valuable assays. Presented herein is an innovative method for detecting functional selectin ligands expressed on human tissue that uses a dynamic approach, which allows for control over the force applied to the bonds between the probe and target molecules. This new method of tissue interrogation, known as dynamic biochemical tissue analysis (DBTA), involves the perfusion of molecular probe-coated microspheres over tissues. DBTA using selectin-coated probes is able to detect functional selectin ligands expressed on tissue from multiple cancer types at both primary and metastatic sites.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Dynamic biochemical tissue analysis detects functional L-selectin ligands on colon cancer tissues.PLoS One. 2017 Mar 10;12(3):e0173747. doi: 10.1371/journal.pone.0173747. eCollection 2017. PLoS One. 2017. PMID: 28282455 Free PMC article.
-
Selectins promote tumor metastasis.Semin Cancer Biol. 2010 Jun;20(3):169-77. doi: 10.1016/j.semcancer.2010.04.005. Epub 2010 May 7. Semin Cancer Biol. 2010. PMID: 20452433 Review.
-
Biophysics of selectin-ligand interactions in inflammation and cancer.Phys Biol. 2011 Feb;8(1):015013. doi: 10.1088/1478-3975/8/1/015013. Epub 2011 Feb 7. Phys Biol. 2011. PMID: 21301059
-
Over-sulfated glycosaminoglycans are alternative selectin ligands: insights into molecular interactions and possible role in breast cancer metastasis.Clin Exp Metastasis. 2013 Oct;30(7):919-31. doi: 10.1007/s10585-013-9592-7. Epub 2013 Jun 6. Clin Exp Metastasis. 2013. PMID: 23739843
-
Targeting selectins and selectin ligands in inflammation and cancer.Expert Opin Ther Targets. 2007 Nov;11(11):1473-91. doi: 10.1517/14728222.11.11.1473. Expert Opin Ther Targets. 2007. PMID: 18028011 Free PMC article. Review.
Cited by
-
Cholesterol depletion decreases adhesion of non-small cell lung cancer cells to E-selectin.Am J Physiol Cell Physiol. 2023 Aug 1;325(2):C471-C482. doi: 10.1152/ajpcell.00197.2020. Epub 2023 Jul 3. Am J Physiol Cell Physiol. 2023. PMID: 37399498 Free PMC article.
-
Sticking to the Problem: Engineering Adhesion in Molecular Endoscopic Imaging.Cell Mol Bioeng. 2020 Jan 21;13(2):113-124. doi: 10.1007/s12195-020-00609-0. eCollection 2020 Apr. Cell Mol Bioeng. 2020. PMID: 32175025 Free PMC article. Review.
References
-
- Köhler S, Ullrich S, Richter U, Schumacher U. E-/P-selectins and colon carcinoma metastasis: first in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br. J. Cancer. 2010;102:602–609. doi: 10.1038/sj.bjc.6605492. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

