The Role of Nonerythroid Spectrin α II in Cancer
- PMID: 31186638
- PMCID: PMC6521328
- DOI: 10.1155/2019/7079604
The Role of Nonerythroid Spectrin α II in Cancer
Abstract
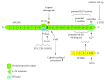
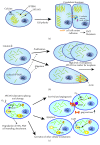
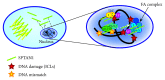
Nonerythroid spectrin αII (SPTAN1) is an important cytoskeletal protein that ensures vital cellular properties including polarity and cell stabilization. In addition, it is involved in cell adhesion, cell-cell contact, and apoptosis. The detection of altered expression of SPTAN1 in tumors indicates that SPTAN1 might be involved in the development and progression of cancer. SPTAN1 has been described in cancer and therapy response and proposed as a potential marker protein for neoplasia, tumor aggressiveness, and therapeutic efficiency. On one hand, the existing data suggest that overexpression of SPTAN1 in tumor cells reflects neoplastic and tumor promoting activity. On the other hand, nuclear SPTAN1 can have tumor suppressing effects by enabling DNA repair through interaction with DNA repair proteins. Moreover, SPTAN1 cleavage products occur during apoptosis and could serve as markers for the efficacy of cancer therapy. Due to SPTAN1's multifaceted functions and its role in adhesion and migration, SPTAN1 can influence tumor growth and progression in both positive and negative directions depending on its specific regulation. This review summarizes the current knowledge on SPTAN1 in cancer and depicts several mechanisms by which SPTAN1 could impact tumor development and aggressiveness.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

