Liquid biopsy for lung cancer immunotherapy
- PMID: 31186680
- PMCID: PMC6507432
- DOI: 10.3892/ol.2019.10166
Liquid biopsy for lung cancer immunotherapy
Abstract
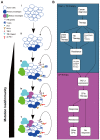
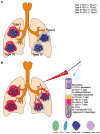
The recent successful use of the immune checkpoint inhibitors (CPIs) anti-programmed death receptor-1 (PD-1)/PD-1 ligand 1 in clinical trials indicates their crucial role in obtaining an effective cancer immune therapy. These CPIs have been identified to have an effective therapeutic response, particularly in tumors with high tumor mutation burden. Targeting private somatic mutations encoding immunogenic neoantigens (neo-Ags) has been developed as an autologous gene therapy. T-cell receptor-engineered T cells targeting neo-Ags are a novel option for adoptive cell therapy used for the treatment of lung cancer. However, not all patients experience an effective response from immunotherapy. Although the resistance mechanism of CPIs has been reported, its association with other treatment methods during systemic anticancer therapy remains unclear, particularly the treatment options following the emergence of drug resistance in lung cancer. The potential biomarkers used for liquid biopsy may assist in the identification of patients who would benefit the most from immunotherapy. Attempts to identify potential biomarkers for predicting clinical response to immunotherapy are underway. With regard to liquid biopsy, the present review summarizes and discusses the lung cancer management of immunotherapy for precision medicine by reviewing recent literature and associated clinical trials.
Keywords: T cells; checkpoint inhibitors; liquid biopsy; lung cancer; neoantigens; prognosis; tumor mutation burden.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
