Efficacy of an activity monitor as a biofeedback device in cerebral palsy
- PMID: 31186915
- PMCID: PMC6453041
- DOI: 10.1177/2055668316676032
Efficacy of an activity monitor as a biofeedback device in cerebral palsy
Abstract
Background: Assessment of physical outcomes in patients with cerebral palsy (CP) is considered an effective way to monitor their progress, evaluate interventions and guide health care policy. However, no study using an activity monitor (AM) as a biofeedback device in treatment of people with CP has been published. Hence, the objective of this study was to evaluate the use of the AM as a biofeedback device in individuals with CP after a type of single-event multilevel surgery (SEMLS) called Single-Event Multilevel Lever Arm Restoration and Anti-Spasticity Surgery (SEMLARASS).
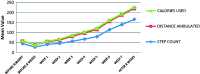
Method: A randomized, controlled trial was conducted among 40 individuals with CP in the age group between 10 to 20 years who underwent SEMLARASS. They were randomly assigned to two groups: Group A (n = 20) and Group B (n = 20). Both groups received intensive rehabilitation including different types of activity-based training for around three hours per day. Both groups were assessed with standard subjective outcomes (Physician Rating Scale (PRS), Dynamic Gait Index (DGI) and Functional Mobility Scale (FMS)) and objective outcomes with the use of an AM (IntenzLife, Model No. 56084-1) for measuring step count, distance walked and calories used. During the intervention, for Group A, they were also given an AM after presetting individualized stride length and body weight, to monitor their daily activity levels. The AM was worn around the neck of the person throughout the day and provided a daily report of the measurements and acted as a biofeedback device for individuals with CP who were given specific targets to achieve on a weekly basis. Both groups were evaluated before and after eight weeks of intensive rehabilitation.
Results: Group A showed significant differences in the scores of step count (p < 0.001), distance walked (p < 0.001), PRS (p < 0.001), DGI (p < 0.010) and FMS (p < 0.001) when compared to Group B after intensive rehabilitation. However, the calories used (p < 0.086) was not significantly different.
Conclusion: The AM, which is considered to be a valid and reliable tool for assessing the level of physical activity in CP, can also be used as a biofeedback device for improving specific walking parameters in persons with CP post-SEMLARASS.
Keywords: Activity monitor; SEMLARASS; SEMLS; biofeedback; cerebral palsy; mobile health devices; wearable devices.
Conflict of interest statement
None declared.
Figures
References
-
- Rosenbaum P, Paneth N, Leviton A, et al. A report: The definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 2007; 109: 8–14. - PubMed
-
- Stanley F, Blair E. Epidemiology and causal pathways. In: Clinics in developmental medicine 2000; Volume 1, London: MacKeith Press.
-
- Blair E, Watson L, Badawi N, et al. Life expectancy among people with cerebral palsy in Western Australia. Dev Med Child Neurol 2001; 43: 508–515. - PubMed
-
- Brehm MA, Becher J, Harlaar J. Reproducibility evaluation of gross and net walking efficiency in children with cerebral palsy. Dev Med Child Neurol 2001; 49: 45–48. - PubMed
-
- Dallmeijer AJ, Brehm MA. Physical strain of comfortable walking in children with mild cerebral palsy. Disabil Rehabil 2011; 33: 1351–1357. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous