Development of a Practice Standard for Monitoring Adult Patients Receiving Bone-Modifying Agents at a Community Cancer Center
- PMID: 31186982
- PMCID: PMC6505660
Development of a Practice Standard for Monitoring Adult Patients Receiving Bone-Modifying Agents at a Community Cancer Center
Abstract
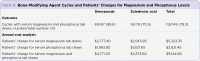
The purpose of this study is to develop a standard for monitoring outpatients starting bone-modifying agents (BMAs) at a community cancer center. The National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) guidelines recommend the monitoring of serum magnesium and phosphorus during BMA therapy but do not define a standard interval. The risk of hypomagnesemia and hypophosphatemia was assessed for the BMAs denosumab, pamidronate, and zoledronic acid. Compliance with dental clearance was also evaluated. Adult cancer outpatients newly started on BMAs between January 1, 2016, to December 31, 2016, were evaluated. Patients with hypercalcemia of malignancy were excluded. Primary endpoints were the composite rates of grade 3 and 4 hypomagnesemia and hypophosphatemia. Secondary endpoints included all-grade hypomagnesemia, all-grade hypophosphatemia, charges for laboratory draws, rate of dental clearance, and rate of osteonecrosis of the jaw (ONJ). Among 61 patients, 4.3% experienced grade 3 and 4 hypophosphatemia. No cases of grade 3 and 4 hypomagnesemia occurred. The annual cost for serum magnesium and phosphorus lab draws totaled $9,144.80. Dental clearance was obtained in 100% of patients, with 67% of clearances obtained from a dentist. No patients developed ONJ. Composite rates of grade 3 and 4 hypomagnesemia and hypophosphatemia were lower than reported in the literature. We propose to monitor magnesium and phosphorus levels at baseline, and then every 6 months. More frequent laboratory draws may be indicated based on clinical judgment. This recommendation will reduce laboratory draws and provide cost savings for patients. Compliance with dental clearance was fully achieved.
Figures




References
-
- Amgen, Inc. Xgeva (denosumab) package insert. 2010 Retrieved from http://pi.amgen.com/united_states/xgeva/xgeva_pi.pdf.
-
- Ben Venue Laboratories, Inc. Pamidronate package insert. 1991
-
- Centers for Disease Control and Prevention. Defining adult overweight and obesity. 2016 Retrieved from https://www.cdc.gov/obesity/adult/defining.html.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
