Randomized Placebo-Controlled Trial Evaluating the Ophthalmic Safety of Single-Dose Tafenoquine in Healthy Volunteers
- PMID: 31187437
- PMCID: PMC6689320
- DOI: 10.1007/s40264-019-00839-w
Randomized Placebo-Controlled Trial Evaluating the Ophthalmic Safety of Single-Dose Tafenoquine in Healthy Volunteers
Abstract
Introduction: Tafenoquine has been recently registered for the prevention of relapse in Plasmodium vivax malaria.
Objective: This study assessed the pharmacodynamic effects of 300-mg single-dose tafenoquine on the retina.
Methods: This phase I, prospective, multicenter, randomized, single-masked, placebo-controlled, parallel-group study was conducted between 2 February 2016 and 14 September 2017 at three US study centers. Adult healthy volunteers were randomized (2:1) to receive either a single 300-mg oral dose of tafenoquine or matched placebo on day 1. Ophthalmic assessments, including spectral domain optical coherence tomography (SD-OCT) and fundus autofluorescence (FAF), were conducted at baseline and day 90 and evaluated for pre-determined endpoints by an independent, masked reading center.
Results: One subject in each group met the composite primary endpoint for retinal changes identified with SD-OCT or FAF, i.e., one out of 306 (0.3%) with tafenoquine, one out of 161 (0.6%) with placebo. Both cases had unilateral focal ellipsoid zone disruption at day 90 with no effect on best-corrected visual acuity. The tafenoquine-treated subject had this abnormality at baseline, and was enrolled in error. There was no difference in ophthalmic safety between tafenoquine and placebo.
Conclusion: There was no evidence of any pharmacodynamic effect of 300-mg single-dose tafenoquine on the retina or any short-term clinically relevant effects on ophthalmic safety. This clinical trial is registered with ClinicalTrials.gov (identifier: NCT02658435).
Conflict of interest statement
Jessica Ackert, Khadeeja Mohamed, Alessandro Berni, Elizabeth Hardaker, Siôn W. Jones, Gavin C.K.W Koh, Jyoti Patel, John Tonkyn, Allen Wolstenholme, and Justin A. Green are employees of GlaxoSmithKline and hold shares in the company. Jason S. Slakter, Hakop Gevorkyan, Hanna Coleman, and Alex Yuan are employees of DARC. Hakop Gevorkyan is an employee of California Clinical Trials Medical Group, and Azra Hussaini is an employee of PAREXEL (California Clinical Trials Medical Group in association with PAREXEL International were contracted and reimbursed for conducting this study). Sherif El-Harazi is an employee of the Lugene Eye Institute; Scott Rasmussen and Keith A. Warren are employees of IQVIA; Deborah S. Kelly is a former employee of GSK and a current employee of Spark Therapeutics; David E. Barañano, John T. Thompson, and Robert C. Sergott received research funding for participating in this study, but have no other relevant conflicts. Robert C. Sergott is an employee of the Wills Eye Hospital. Stephan Duparc is an employee of Medicines for Malaria Venture.
Figures

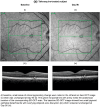


References
-
- Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383(9922):1049–1058. doi: 10.1016/S0140-6736(13)62568-4. - DOI - PubMed
-
- World Health Organization. World Malaria Report. WHO, Geneva. 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/repor.... Accessed 22 Nov 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

