Lumiflavin increases the sensitivity of ovarian cancer stem-like cells to cisplatin by interfering with riboflavin
- PMID: 31187586
- PMCID: PMC6652702
- DOI: 10.1111/jcmm.14409
Lumiflavin increases the sensitivity of ovarian cancer stem-like cells to cisplatin by interfering with riboflavin
Abstract
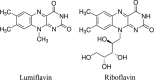
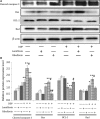
Here, we used lumiflavin, an inhibitor of riboflavin, as a new potential therapeutic chemosensitizer to ovarian cancer stem-like cells (CSCs). This study demonstrates that the enrichment of riboflavin in CSCs is an important cause of its resistance to chemotherapy. Lumiflavin can effectively reduce the riboflavin enrichment in CSCs and sensitize the effect of cisplatin Diamminedichloroplatinum (DDP) on CSCs. In this study, CSCs of human ovarian cancer cell lines HO8910 were separated using a magnetic bead (CD133+). We also show the overexpression of the mRNA and protein of riboflavin transporter 2 and the high content of riboflavin in CSCs compared to non-CSCs (NON-CSCs). Moreover, CSCs were less sensitive to DDP than NON-CSCs, whereas, the synergistic effect of lumiflavin and DDP on CSCs was more sensitive than NON-CSCs. Further research showed that lumiflavin had synergistic effects with DDP on CSCs in increasing mitochondrial function damage and apoptosis rates and decreasing clonic function. In addition, we found that the combination of DDP and lumiflavin therapy in vivo has a synergistic cytotoxic effect on an ovarian cancer nude mice model by enhancing the DNA-damage response and increasing the apoptotic protein expression. Notably, the effect of lumiflavin is associated with reduced riboflavin concentration, and riboflavin could reverse the effect of DDP in vitro and in vivo. Accordingly, we conclude that lumiflavin interfered with the riboflavin metabolic pathways, resulting in a significant increase in tumour sensitivity to DDP therapy. Our study suggests that lumiflavin may be a novel treatment alternative for ovarian cancer and its recurrence.
Keywords: cancer stem-like cells; cisplatin; lumiflavin; ovarian cancer; riboflavin.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors confirm that there is no conflict of interest.
Figures




Similar articles
-
Lumiflavin Reduces Cisplatin Resistance in Cancer Stem-Like Cells of OVCAR-3 Cell Line by Inducing Differentiation.Front Oncol. 2022 May 20;12:859275. doi: 10.3389/fonc.2022.859275. eCollection 2022. Front Oncol. 2022. PMID: 35669418 Free PMC article.
-
Lumiflavin Enhances the Effects of Ionising Radiation on Ovarian Cancer Stem-Like Cells by Inhibiting Autophagy.Anticancer Agents Med Chem. 2021;21(15):2004-2011. doi: 10.2174/1871520621999210104201907. Anticancer Agents Med Chem. 2021. PMID: 33397270
-
Noscapine Increases the Sensitivity of Drug-Resistant Ovarian Cancer Cell Line SKOV3/DDP to Cisplatin by Regulating Cell Cycle and Activating Apoptotic Pathways.Cell Biochem Biophys. 2015 May;72(1):203-13. doi: 10.1007/s12013-014-0438-y. Cell Biochem Biophys. 2015. PMID: 25510462
-
Therapeutic strategies to overcome cisplatin resistance in ovarian cancer.Eur J Med Chem. 2022 Mar 15;232:114205. doi: 10.1016/j.ejmech.2022.114205. Epub 2022 Feb 17. Eur J Med Chem. 2022. PMID: 35217497 Review.
-
Mitochondrial integration and ovarian cancer chemotherapy resistance.Exp Cell Res. 2021 Apr 15;401(2):112549. doi: 10.1016/j.yexcr.2021.112549. Epub 2021 Feb 26. Exp Cell Res. 2021. PMID: 33640393 Review.
Cited by
-
Lumiflavin Reduces Cisplatin Resistance in Cancer Stem-Like Cells of OVCAR-3 Cell Line by Inducing Differentiation.Front Oncol. 2022 May 20;12:859275. doi: 10.3389/fonc.2022.859275. eCollection 2022. Front Oncol. 2022. PMID: 35669418 Free PMC article.
-
LncRNA WDFY3-AS2 promotes cisplatin resistance and the cancer stem cell in ovarian cancer by regulating hsa-miR-139-5p/SDC4 axis.Cancer Cell Int. 2021 May 29;21(1):284. doi: 10.1186/s12935-021-01993-x. Cancer Cell Int. 2021. PMID: 34051810 Free PMC article.
References
-
- Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM. A brief review of the management of platinum‐resistant–platinum‐refractory ovarian cancer. Med Oncol. 2017;34:103. - PubMed
-
- Cojoc M, Mäbert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Seminars in cancer biology. Elsevier. 2015;16‐27. - PubMed
-
- Miranda‐Lorenzo I, Dorado J, Lonardo E, et al. Intracellular autofluorescence: a biomarker for epithelial cancer stem cells. Nat Methods. 2014;11:1161. - PubMed
-
- Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181‐2196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

