A counter gradient of Activin A and follistatin instructs the timing of hair cell differentiation in the murine cochlea
- PMID: 31187730
- PMCID: PMC6561706
- DOI: 10.7554/eLife.47613
A counter gradient of Activin A and follistatin instructs the timing of hair cell differentiation in the murine cochlea
Abstract
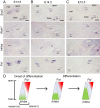
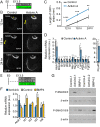
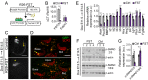
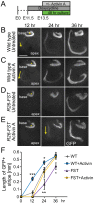
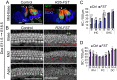
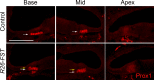
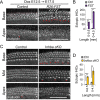
The mammalian auditory sensory epithelium has one of the most stereotyped cellular patterns known in vertebrates. Mechano-sensory hair cells are arranged in precise rows, with one row of inner and three rows of outer hair cells spanning the length of the spiral-shaped sensory epithelium. Aiding such precise cellular patterning, differentiation of the auditory sensory epithelium is precisely timed and follows a steep longitudinal gradient. The molecular signals that promote auditory sensory differentiation and instruct its graded pattern are largely unknown. Here, we identify Activin A and its antagonist follistatin as key regulators of hair cell differentiation and show, using mouse genetic approaches, that a local gradient of Activin A signaling within the auditory sensory epithelium times the longitudinal gradient of hair cell differentiation. Furthermore, we provide evidence that Activin-type signaling regulates a radial gradient of terminal mitosis within the auditory sensory epithelium, which constitutes a novel mechanism for limiting the number of inner hair cells being produced.
Keywords: activin signaling; auditory cell differentiation; cochlea; developmental biology; follistatin; hair cell; inner ear; mouse; neuroscience.
© 2019, Prajapati-DiNubila et al.
Conflict of interest statement
MP, AB, EG, SZ, AD No competing interests declared
Figures
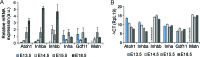

References
-
- Basch ML, Brown RM, Jen HI, Semerci F, Depreux F, Edlund RK, Zhang H, Norton CR, Gridley T, Cole SE, Doetzlhofer A, Maletic-Savatic M, Segil N, Groves AK. Fine-tuning of notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. eLife. 2016;5:e19921. doi: 10.7554/eLife.19921. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

