Tissue Engineering of the Microvasculature
- PMID: 31187896
- PMCID: PMC7025285
- DOI: 10.1002/cphy.c180037
Tissue Engineering of the Microvasculature
Abstract
The ability to generate new microvessels in desired numbers and at desired locations has been a long-sought goal in vascular medicine, engineering, and biology. Historically, the need to revascularize ischemic tissues nonsurgically (so-called therapeutic vascularization) served as the main driving force for the development of new methods of vascular growth. More recently, vascularization of engineered tissues and the generation of vascularized microphysiological systems have provided additional targets for these methods, and have required adaptation of therapeutic vascularization to biomaterial scaffolds and to microscale devices. Three complementary strategies have been investigated to engineer microvasculature: angiogenesis (the sprouting of existing vessels), vasculogenesis (the coalescence of adult or progenitor cells into vessels), and microfluidics (the vascularization of scaffolds that possess the open geometry of microvascular networks). Over the past several decades, vascularization techniques have grown tremendously in sophistication, from the crude implantation of arteries into myocardial tunnels by Vineberg in the 1940s, to the current use of micropatterning techniques to control the exact shape and placement of vessels within a scaffold. This review provides a broad historical view of methods to engineer the microvasculature, and offers a common framework for organizing and analyzing the numerous studies in this area of tissue engineering and regenerative medicine. © 2019 American Physiological Society. Compr Physiol 9:1155-1212, 2019.
Copyright © 2019 American Physiological Society. All rights reserved.
Figures
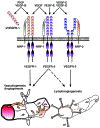

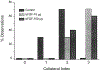


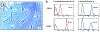
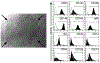


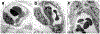

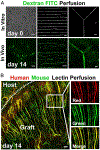







References
-
- Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation 107: 2134–2139, 2003. - PubMed
-
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9: 1370–1376, 2003. - PubMed
-
- Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res 100: 581–589, 2007. - PubMed
-
- Akahori T, Kobayashi A, Komaki M, Hattori H, Nakahama K-I, Ichinose S, Abe M, Takeda S, Morita I. Implantation of capillary structure engineered by optical lithography improves hind limb ischemia in mice. Tissue Eng A 16: 953–959, 2010. - PubMed
-
- Akita S, Tamai N, Myoui A, Nishikawa M, Kaito T, Takaoka K, Yoshikawa H. Capillary vessel network integration by inserting a vascular pedicle enhances bone formation in tissue-engineered bone using interconnected porous hydroxyapatite ceramics. Tissue Eng 10: 789–795, 2004. - PubMed
FURTHER READING
-
- Briquez PS, Clegg LE, Martino MM, Mac Gabhann F, Hubbell JA. Design principles for therapeutic angiogenic materials. Nat Rev Mater 1: 1–15, 2016.
-
- Lähteenvuo J, Ylä-Herttuala S. Advances and challenges in cardiovascular gene therapy. Hum Gene Ther 28: 1024–1032, 2017. - PubMed
CROSS-REFERENCES
-
-
Cardiovascular Physiology: Overview of the microcirculation (legacy)
-
-
-
Cardiovascular Physiology: Physiology and pathobiology of microvascular endothelium (legacy)
-
-
-
Cardiovascular Physiology: Angiogenesis
-
-
-
Cardiovascular Physiology: Development of a vascular network
-
-
-
Cardiovascular Physiology: Lymphatics
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

