Metal-responsive RNA polymerase extracytoplasmic function (ECF) sigma factors
- PMID: 31187912
- PMCID: PMC6851896
- DOI: 10.1111/mmi.14328
Metal-responsive RNA polymerase extracytoplasmic function (ECF) sigma factors
Abstract
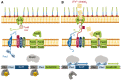
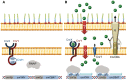
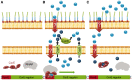
In order to survive, bacteria must adapt to multiple fluctuations in their environment, including coping with changes in metal concentrations. Many metals are essential for viability, since they act as cofactors of indispensable enzymes. But on the other hand, they are potentially toxic because they generate reactive oxygen species or displace other metals from proteins, turning them inactive. This dual effect of metals forces cells to maintain homeostasis using a variety of systems to import and export them. These systems are usually inducible, and their expression is regulated by metal sensors and signal-transduction mechanisms, one of which is mediated by extracytoplasmic function (ECF) sigma factors. In this review, we have focused on the metal-responsive ECF sigma factors, several of which are activated by iron depletion (FecI, FpvI and PvdS), while others are activated by excess of metals such as nickel and cobalt (CnrH), copper (CarQ and CorE) or cadmium and zinc (CorE2). We focus particularly on their physiological roles, mechanisms of action and signal transduction pathways.
© 2019 The Authors. Molecular Microbiology Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mechanisms of Action of Non-Canonical ECF Sigma Factors.Int J Mol Sci. 2022 Mar 25;23(7):3601. doi: 10.3390/ijms23073601. Int J Mol Sci. 2022. PMID: 35408957 Free PMC article. Review.
-
Contribution of extracytoplasmic function sigma factors to transition metal homeostasis in Cupriavidus metallidurans strain CH34.J Mol Microbiol Biotechnol. 2007;12(3-4):227-40. doi: 10.1159/000099644. J Mol Microbiol Biotechnol. 2007. PMID: 17587871
-
ECF σ factors with regulatory extensions: the one-component systems of the σ universe.Mol Microbiol. 2019 Aug;112(2):399-409. doi: 10.1111/mmi.14323. Epub 2019 Jun 26. Mol Microbiol. 2019. PMID: 31175685 Review.
-
The third pillar of metal homeostasis in Cupriavidus metallidurans CH34: preferences are controlled by extracytoplasmic function sigma factors.Metallomics. 2019 Feb 20;11(2):291-316. doi: 10.1039/c8mt00299a. Metallomics. 2019. PMID: 30681120
-
Characterization of an ECF sigma factor protein from Pseudomonas aeruginosa.Biochem Biophys Res Commun. 2000 Jul 5;273(2):578-83. doi: 10.1006/bbrc.2000.2996. Biochem Biophys Res Commun. 2000. PMID: 10873648
Cited by
-
The role of transcriptional regulators in metal ion homeostasis of Mycobacterium tuberculosis.Front Cell Infect Microbiol. 2024 Mar 11;14:1360880. doi: 10.3389/fcimb.2024.1360880. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38529472 Free PMC article. Review.
-
Physiological Roles of an Acinetobacter-specific σ Factor.bioRxiv [Preprint]. 2024 Jul 8:2024.07.08.602572. doi: 10.1101/2024.07.08.602572. bioRxiv. 2024. Update in: mBio. 2025 Jun 11;16(6):e0096825. doi: 10.1128/mbio.00968-25. PMID: 39026751 Free PMC article. Updated. Preprint.
-
The bacterial iron sensor IdeR recognizes its DNA targets by indirect readout.Nucleic Acids Res. 2021 Sep 27;49(17):10120-10135. doi: 10.1093/nar/gkab711. Nucleic Acids Res. 2021. PMID: 34417623 Free PMC article.
-
Multiple Mechanisms for Copper Uptake by Methylosinus trichosporium OB3b in the Presence of Heterologous Methanobactin.mBio. 2022 Oct 26;13(5):e0223922. doi: 10.1128/mbio.02239-22. Epub 2022 Sep 21. mBio. 2022. PMID: 36129259 Free PMC article.
-
Mechanisms of Action of Non-Canonical ECF Sigma Factors.Int J Mol Sci. 2022 Mar 25;23(7):3601. doi: 10.3390/ijms23073601. Int J Mol Sci. 2022. PMID: 35408957 Free PMC article. Review.
References
-
- Abellón‐Ruiz, J. , Bernal‐Bernal, D. , Abellan, M. , Fontes, M. , Padmanabhan, S. , Murillo, F.J. and Elías‐Arnanz, M. (2014) The CarD/CarG regulatory complex is required for the action of several members of the large set of Myxococcus xanthus extracytoplasmic function σ factors. Environmental Microbiology, 16, 2475–2490. - PubMed
-
- Angerer, A. , Enz, S. , Ochs, M. and Braun, V. (1995) Transcriptional regulation of ferric citrate transport in Escherichia coli K‐12. FecI belongs to a new subfamily of σ70‐type factors that respond to extracytoplasmic stimuli. Molecular Microbiology, 18, 163–174. - PubMed
-
- Barras, F. and Fontecave, M. (2011) Cobalt stress in Escherichia coli and Salmonella enterica: molecular bases for toxicity and resistance. Metallomics, 3, 1130–1134. - PubMed
-
- Beare, P.A. , For, R.J. , Martin, L.W. and Lamont, I.L. (2003) Siderophore‐mediated cell signalling in Pseudomonas aeruginosa: divergent pathways regulate virulence factor production and siderophore receptor synthesis. Molecular Microbiology, 47, 195–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

