Omission of temporal nuisance regressors from dual regression can improve accuracy of fMRI functional connectivity maps
- PMID: 31187917
- PMCID: PMC6865788
- DOI: 10.1002/hbm.24692
Omission of temporal nuisance regressors from dual regression can improve accuracy of fMRI functional connectivity maps
Abstract
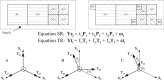
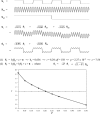
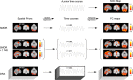
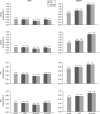
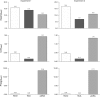
Functional connectivity (FC) maps from brain fMRI data can be derived with dual regression, a proposed alternative to traditional seed-based FC (SFC) methods that detect temporal correlation between a predefined region (seed) and other regions in the brain. As with SFC, incorporating nuisance regressors (NR) into the dual regression must be done carefully, to prevent potential bias and insensitivity of FC estimates. Here, we explore the potentially untoward effects on dual regression that may occur when NR correlate highly with the signal of interest, using both synthetic and real fMRI data to elucidate mechanisms responsible for loss of accuracy in FC maps. Our tests suggest significantly improved accuracy in FC maps derived with dual regression when highly correlated temporal NR were omitted. Single-map dual regression, a simplified form of dual regression that uses neither spatial nor temporal NR, offers a viable alternative whose FC maps may be more easily interpreted, and in some cases be more accurate than those derived with standard dual regression.
Keywords: brain mapping; functional neuroimaging; image enhancement; investigative techniques; magnetic resonance imaging.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no competing conflict of interest.
Figures





Similar articles
-
Seed-based dual regression: An illustration of the impact of dual regression's inherent filtering of global signal.J Neurosci Methods. 2022 Jan 15;366:109410. doi: 10.1016/j.jneumeth.2021.109410. Epub 2021 Nov 16. J Neurosci Methods. 2022. PMID: 34798212 Free PMC article.
-
Hybrid ICA-Seed-Based Methods for fMRI Functional Connectivity Assessment: A Feasibility Study.Int J Biomed Imaging. 2010;2010:868976. doi: 10.1155/2010/868976. Epub 2010 Jun 28. Int J Biomed Imaging. 2010. PMID: 20689712 Free PMC article.
-
Nuisance effects and the limitations of nuisance regression in dynamic functional connectivity fMRI.Neuroimage. 2019 Jan 1;184:1005-1031. doi: 10.1016/j.neuroimage.2018.09.024. Epub 2018 Sep 14. Neuroimage. 2019. PMID: 30223062 Free PMC article.
-
Noise Reduction in Arterial Spin Labeling Based Functional Connectivity Using Nuisance Variables.Front Neurosci. 2016 Aug 23;10:371. doi: 10.3389/fnins.2016.00371. eCollection 2016. Front Neurosci. 2016. PMID: 27601973 Free PMC article.
-
Nuisance effects in inter-scan functional connectivity estimates before and after nuisance regression.Neuroimage. 2019 Nov 15;202:116005. doi: 10.1016/j.neuroimage.2019.07.018. Epub 2019 Jul 20. Neuroimage. 2019. PMID: 31336189 Free PMC article.
Cited by
-
Seed-based dual regression: An illustration of the impact of dual regression's inherent filtering of global signal.J Neurosci Methods. 2022 Jan 15;366:109410. doi: 10.1016/j.jneumeth.2021.109410. Epub 2021 Nov 16. J Neurosci Methods. 2022. PMID: 34798212 Free PMC article.
References
-
- Aguirre, G. K. , Zarahn, E. , & D'Esposito, M. (1998). The inferential impact of global signal covariates in functional neuroimaging analyses. NeuroImage, 8, 302–306. - PubMed
-
- Ardekani, B. A. , Guckemus, S. , Bachman, A. , Hoptman, M. J. , Wojtaszek, M. , & Nierenberg, J. (2005). Quantitative comparison of algorithms for inter‐subject registration of 3D volumetric brain MRI scans. Journal of Neuroscience Methods, 142, 67–76. - PubMed
Publication types
Grants and funding
- U01 MH062518/MH/NIMH NIH HHS/United States
- MH064783/GF/NIH HHS/United States
- MH062518/GF/NIH HHS/United States
- MH102252/GF/NIH HHS/United States
- R01 MH064783/MH/NIMH NIH HHS/United States
- R21 MH084031/MH/NIMH NIH HHS/United States
- MH096685/GF/NIH HHS/United States
- MH097735/GF/NIH HHS/United States
- R01 MH096685/MH/NIMH NIH HHS/United States
- MH084031/GF/NIH HHS/United States
- R01 MH102252/MH/NIMH NIH HHS/United States
- R01 MH097735/MH/NIMH NIH HHS/United States
- T32 MH019132/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

