Regulation of renal NaDC1 expression and citrate excretion by NBCe1-A
- PMID: 31188034
- PMCID: PMC6732450
- DOI: 10.1152/ajprenal.00015.2019
Regulation of renal NaDC1 expression and citrate excretion by NBCe1-A
Abstract
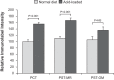
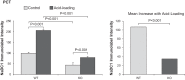
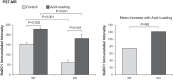
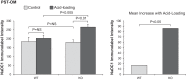
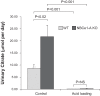
Citrate is critical for acid-base homeostasis and to prevent calcium nephrolithiasis. Both metabolic acidosis and hypokalemia decrease citrate excretion and increase expression of Na+-dicarboxylate cotransporter 1 (NaDC1; SLC13A2), the primary protein involved in citrate reabsorption. However, the mechanisms transducing extracellular signals and mediating these responses are incompletely understood. The purpose of the present study was to determine the role of the Na+-coupled electrogenic bicarbonate cotransporter (NBCe1) A variant (NBCe1-A) in citrate metabolism under basal conditions and in response to acid loading and hypokalemia. NBCe1-A deletion increased citrate excretion and decreased NaDC1 expression in the proximal convoluted tubules (PCT) and proximal straight tubules (PST) in the medullary ray (PST-MR) but not in the PST in the outer medulla (PST-OM). Acid loading wild-type (WT) mice decreased citrate excretion. NaDC1 expression increased only in the PCT and PST-MR and not in the PST-MR. In NBCe1-A knockout (KO) mice, the acid loading change in citrate excretion was unaffected, changes in PCT NaDC1 expression were blocked, and there was an adaptive increase in PST-MR. Hypokalemia in WT mice decreased citrate excretion; NaDC1 expression increased only in the PCT and PST-MR. NBCe1-A KO blocked both the citrate and NaDC1 changes. We conclude that 1) adaptive changes in NaDC1 expression in response to metabolic acidosis and hypokalemia occur specifically in the PCT and PST-MR, i.e., in cortical proximal tubule segments; 2) NBCe1-A is necessary for normal basal, metabolic acidosis and hypokalemia-stimulated citrate metabolism and does so by regulating NaDC1 expression in cortical proximal tubule segments; and 3) adaptive increases in PST-OM NaDC1 expression occur in NBCe1-A KO mice in response to acid loading that do not occur in WT mice.
Keywords: Na+-dicarboxylate cotransporter 1; citrate; proximal tubule.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures







References
-
- Alper SL. Familial renal tubular acidosis. J Nephrol 23, Suppl 16: S57–S76, 2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

