A reversible autophagy inhibitor blocks autophagosome-lysosome fusion by preventing Stx17 loading onto autophagosomes
- PMID: 31188703
- PMCID: PMC6743457
- DOI: 10.1091/mbc.E18-08-0482
A reversible autophagy inhibitor blocks autophagosome-lysosome fusion by preventing Stx17 loading onto autophagosomes
Abstract
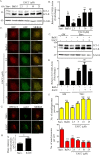
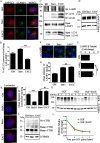
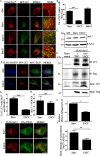
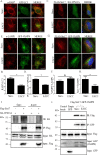
Autophagy is an evolutionarily conserved intracellular lysosomal degradation pathway. It is a multistep process involving de novo formation of double membrane autophagosomes that capture cytosolic constituents (cargo) and eventually fuse with lysosomes wherein the cargo gets degraded and resulting simpler biomolecules get recycled. In addition to their autophagy function, several of the autophagy-related proteins work at the interface of other vesicular trafficking pathways. Hence, development of specific autophagy modulators that do not perturb general endo-lysosomal traffic possesses unique challenges. In this article, we report a novel small molecule EACC that inhibits autophagic flux by blocking autophagosome-lysosome fusion. Strikingly, unlike other late stage inhibitors, EACC does not have any effect on lysosomal properties or on endocytosis-mediated degradation of EGF receptor. EACC affects the translocation of SNAREs Stx17 and SNAP29 on autophagosomes without impeding the completion of autophagosomes. EACC treatment also reduces the interaction of Stx17 with the HOPS subunit VPS33A and the cognate lysosomal R-SNARE VAMP8. Interestingly, this effect of EACC although quite robust is reversible and hence EACC can be used as a tool to study autophagosomal SNARE trafficking. Our results put forward a novel method to block autophagic flux by impeding the action of the autophagosomal SNAREs.
Figures


References
-
- Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC. (2016). Mammalian autophagy: how does it work? Annu Rev Biochem , 685–713. - PubMed
-
- Bonifacino JS, Glick BS. (2004). The mechanisms of vesicle budding and fusion. Cell , 153–166. - PubMed
-
- Cai H, Reinisch K, Ferro-Novick S. (2007). Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell , 671–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

