" Candida Albicans Interactions With The Host: Crossing The Intestinal Epithelial Barrier"
- PMID: 31189436
- PMCID: PMC6619947
- DOI: 10.1080/21688370.2019.1612661
" Candida Albicans Interactions With The Host: Crossing The Intestinal Epithelial Barrier"
Abstract
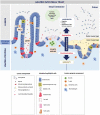
Formerly a commensal organism of the mucosal surfaces of most healthy individuals, Candida albicans is an opportunistic pathogen that causes infections ranging from superficial to the more life-threatening disseminated infections, especially in the ever-growing population of vulnerable patients in the hospital setting. In these situations, the fungus takes advantage of its host following a disturbance in the host defense system and/or the mucosal microbiota. Overwhelming evidence suggests that the gastrointestinal tract is the main source of disseminated C. albicans infections. Major risk factors for disseminated candidiasis include damage to the mucosal intestinal barrier, immune dysfunction, and dysbiosis of the resident microbiota. A better understanding of C. albicans' interaction with the intestinal epithelial barrier will be useful for designing future therapies to avoid systemic candidiasis. In this review, we provide an overview of the current knowledge regarding the mechanisms of pathogenicity that allow the fungus to reach and translocate the gut barrier.
Keywords: Candida albicans; adherence; damage; enterocyte; infection models; interaction; intestinal barrier; invasion.
Figures



References
-
- Tadec L, Talarmin J-P, Gastinne T, Bretonnière C, Miegeville M, Le Pape P, Morio F.. Epidemiology, risk factor, species distribution, antifungal resistance and outcome of Candidemia at a single French hospital: a 7-year study. Mycoses. 2016;59(5):296–303. doi: 10.1111/myc.2016.59.issue-5. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
