AMP-activated protein kinase complexes containing the β2 regulatory subunit are up-regulated during and contribute to adipogenesis
- PMID: 31189568
- PMCID: PMC6595317
- DOI: 10.1042/BCJ20180714
AMP-activated protein kinase complexes containing the β2 regulatory subunit are up-regulated during and contribute to adipogenesis
Abstract
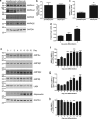
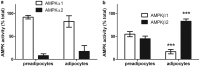
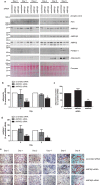
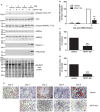
AMP-activated protein kinase (AMPK) is a heterotrimer of α-catalytic and β- and γ-regulatory subunits that acts to regulate cellular and whole-body nutrient metabolism. The key role of AMPK in sensing energy status has led to significant interest in AMPK as a therapeutic target for dysfunctional metabolism in type 2 diabetes, insulin resistance and obesity. Despite the actions of AMPK in the liver and skeletal muscle being extensively studied, the role of AMPK in adipose tissue and adipocytes remains less well characterised. Small molecules that selectively influence AMPK heterotrimers containing specific AMPKβ subunit isoforms have been developed, including MT47-100, which selectively inhibits complexes containing AMPKβ2. AMPKβ1 and AMPKβ2 are the principal AMPKβ subunit isoforms in rodent liver and skeletal muscle, respectively, yet the contribution of specific AMPKβ isoforms to adipose tissue function, however, remains largely unknown. This study therefore sought to determine the contribution of AMPKβ subunit isoforms to adipocyte biology, focussing on adipogenesis. AMPKβ2 was the principal AMPKβ isoform in 3T3-L1 adipocytes, isolated rodent adipocytes and human subcutaneous adipose tissue, as assessed by the contribution to total cellular AMPK activity. Down-regulation of AMPKβ2 with siRNA inhibited lipid accumulation, cellular adiponectin levels and adiponectin secretion during 3T3-L1 adipogenesis, whereas down-regulation of AMPKβ1 had no effect. Incubation of 3T3-L1 cells with MT47-100 selectively inhibited AMPK complexes containing AMPKβ2 whilst simultaneously inhibiting cellular lipid accumulation as well as cellular levels and secretion of adiponectin. Taken together, these data indicate that increased expression of AMPKβ2 is an important feature of efficient adipogenesis.
Keywords: AMPK; adipocytes; adipogenesis.
© 2019 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
HM-chromanone inhibits adipogenesis by regulating adipogenic transcription factors and AMPK in 3T3-L1 adipocytes.Eur J Pharmacol. 2021 Feb 5;892:173689. doi: 10.1016/j.ejphar.2020.173689. Epub 2020 Oct 28. Eur J Pharmacol. 2021. PMID: 33127360
-
Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes.Int J Mol Sci. 2021 Oct 22;22(21):11409. doi: 10.3390/ijms222111409. Int J Mol Sci. 2021. PMID: 34768840 Free PMC article.
-
AMPKβ subunits: more than just a scaffold in the formation of AMPK complex.FEBS J. 2013 Aug;280(16):3723-33. doi: 10.1111/febs.12364. Epub 2013 Jun 24. FEBS J. 2013. PMID: 23721051 Review.
-
Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways.Biomed Pharmacother. 2018 Jun;102:670-680. doi: 10.1016/j.biopha.2018.03.124. Epub 2018 Apr 5. Biomed Pharmacother. 2018. PMID: 29604586
-
Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation.Clin Sci (Lond). 2013 Apr;124(8):491-507. doi: 10.1042/CS20120536. Clin Sci (Lond). 2013. PMID: 23298225 Review.
Cited by
-
WW domain-binding protein 2 overexpression prevents diet-induced liver steatosis and insulin resistance through AMPKβ1.Cell Death Dis. 2021 Mar 3;12(3):228. doi: 10.1038/s41419-021-03536-8. Cell Death Dis. 2021. PMID: 33658485 Free PMC article.
-
AMPKβ isoform expression patterns in various adipocyte models and in relation to body mass index.Front Physiol. 2022 Aug 4;13:928964. doi: 10.3389/fphys.2022.928964. eCollection 2022. Front Physiol. 2022. PMID: 35991175 Free PMC article.
-
Potential antidiabetic activity of benzimidazole derivative albendazole and lansoprazole drugs in different doses in experimental type 2 diabetic rats.Turk J Med Sci. 2021 Jun 28;51(3):1579-1586. doi: 10.3906/sag-2004-38. Turk J Med Sci. 2021. PMID: 33641315 Free PMC article.
-
The metabolic sensor AMPK: Twelve enzymes in one.Mol Metab. 2024 Dec;90:102042. doi: 10.1016/j.molmet.2024.102042. Epub 2024 Oct 2. Mol Metab. 2024. PMID: 39362600 Free PMC article. Review.
-
Research progress of AMP-activated protein kinase and cardiac aging.Open Life Sci. 2023 Aug 29;18(1):20220710. doi: 10.1515/biol-2022-0710. eCollection 2023. Open Life Sci. 2023. PMID: 37671091 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

