Changes in hepatitis C burden and treatment trends in Europe during the era of direct-acting antivirals: a modelling study
- PMID: 31189677
- PMCID: PMC6576109
- DOI: 10.1136/bmjopen-2018-026726
Changes in hepatitis C burden and treatment trends in Europe during the era of direct-acting antivirals: a modelling study
Abstract
Objectives: Oral direct-acting antivirals (DAAs) for hepatitis C virus (HCV) have dramatically changed the treatment paradigm. Our aim was to project temporal trends in HCV diagnosis, treatment and disease burden in France, Germany, Italy, Spain and the UK.
Design: A mathematical simulation model of natural history of HCV infection.
Participants: HCV-infected patients defined based on country-specific age, fibrosis and genotype distributions.
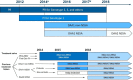
Interventions: HCV screening practice and availability of different waves of DAA treatment in each country.
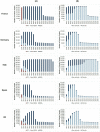
Outcome measures: Temporal trends in the number of patients who achieve sustained virological response (SVR), fail treatment (by drug regimen) and develop advanced sequelae from 2014 to 2030 in each country.
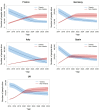
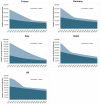
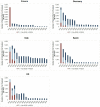
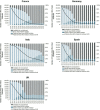
Results: We projected that 1 324 000 individuals would receive treatment from 2014 to 2030 in the five European countries and 12 000-37 000 of them would fail to achieve SVR. By 2021, the number of individuals cured of HCV would supersede the number of actively infected individuals in France, Germany, Spain and the UK. Under status quo, the diagnosis rate would reach between 65% and 75% and treatment coverage between 65% and 74% by 2030 in these countries. The number of patients who fail treatment would decrease over time, with the majority of those who fail treatment having been exposed to non-structural protein 5A inhibitors.
Conclusions: In the era of DAAs, the number of people with HCV who achieved a cure will exceed the number of viraemic patients, but many patients will remain undiagnosed, untreated, fail multiple treatments and develop advanced sequelae. Scaling-up screening and treatment capacity, and timely and effective retreatment are needed to avail the full benefits of DAAs and to meet HCV elimination targets set by WHO.
Keywords: direct-acting antivirals; disease trend; hepatitis C elimination; simulation model; treatment failure.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JC received research grants and consulting fee from Gilead Science and Merck. TA received consulting fee from Gilead Sciences. SF has servered a speaker for Gilead Sciences, MSD, Abbvie, Bristol–Myers Squibb, Novartis, Bayer and Janssen. All other authors have nothing to report.
Figures
References
-
- World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. 2016. http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ (Accessed 3 Jan 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
