Therapeutic Effect of Y-27632 on Tumorigenesis and Cisplatin-Induced Peripheral Sensory Loss through RhoA-NF-κB
- PMID: 31189689
- PMCID: PMC6774348
- DOI: 10.1158/1541-7786.MCR-19-0024
Therapeutic Effect of Y-27632 on Tumorigenesis and Cisplatin-Induced Peripheral Sensory Loss through RhoA-NF-κB
Abstract
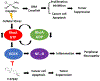
Chemotherapy-induced peripheral neuropathy (CIPN) is a major side effect of cancer therapy that frequently requires a reduction or cessation of treatments and negatively impacts the patient's quality of life. There is currently no effective means to prevent or treat CIPN. In this study, we developed and applied CIPN in an immunocompetent, syngeneic murine Lewis Lung Carcinoma (LLCab) model that enabled the elucidation of both tumor and host responses to cisplatin and treatments of Y-27632, a selective inhibitor of Rho kinase/p160ROCK. Y-27632 not only preserved cisplatin's efficacy toward tumor suppression but also the combination treatment inhibited tumor cell proliferation and increased cellular apoptosis. By alleviating the cisplatin-induced loss of epidermal nerve fibers (ENFs), Y-27632 protected tumor-bearing mice from cisplatin-induced reduction of touch sensation. Furthermore, quantitative proteomic analysis revealed the striking cisplatin-induced dysregulation in cellular stress (inflammation, mitochondrial deficiency, DNA repair, etc.)-associated proteins. Y-27632 was able to reverse the changes of these proteins that are associated with Rho GTPase and NF-κB signaling network, and also decreased cisplatin-induced NF-κB hyperactivation in both footpad tissues and tumor. Therefore, Y-27632 is an effective adjuvant in tumor suppression and peripheral neuroprotection. These studies highlight the potential of targeting the RhoA-NF-κB axis as a combination therapy to treat CIPN. IMPLICATIONS: This study, for the first time, demonstrated the dual antineoplastic and neuroprotective effects of Rho kinase/p160ROCK inhibition in a syngeneic immunocompetent tumor-bearing mouse model, opening the door for further clinical adjuvant development of RhoA-NF-κB axis to improve chemotherapeutic outcomes.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures
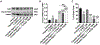
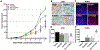
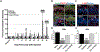

Similar articles
-
Amelioration of cisplatin-induced experimental peripheral neuropathy by a small molecule targeting p75 NTR.Neurotoxicology. 2014 Dec;45:81-90. doi: 10.1016/j.neuro.2014.09.005. Epub 2014 Sep 30. Neurotoxicology. 2014. PMID: 25277379 Free PMC article.
-
Neuroprotective Effects of Trimetazidine against Cisplatin-Induced Peripheral Neuropathy: Involvement of AMPK-Mediated PI3K/mTOR, Nrf2, and NF-κB Signaling Axes.Oxid Med Cell Longev. 2024 Aug 20;2024:6612009. doi: 10.1155/2024/6612009. eCollection 2024. Oxid Med Cell Longev. 2024. PMID: 39502494 Free PMC article.
-
Concurrent blockade of NF-κB and Akt pathways potentiates cisplatin's antitumor activity in vivo.Anticancer Drugs. 2012 Nov;23(10):1039-46. doi: 10.1097/CAD.0b013e32835679b8. Anticancer Drugs. 2012. PMID: 22760211
-
Pro-Inflammatory Signalling PRRopels Cisplatin-Induced Toxicity.Int J Mol Sci. 2022 Jun 29;23(13):7227. doi: 10.3390/ijms23137227. Int J Mol Sci. 2022. PMID: 35806229 Free PMC article. Review.
-
Animal models of cisplatin-induced neuropathic pain.Animal Model Exp Med. 2025 Jan 23. doi: 10.1002/ame2.12548. Online ahead of print. Animal Model Exp Med. 2025. PMID: 39854055 Review.
Cited by
-
New insights on the regulators and inhibitors of RhoA-ROCK signalling in Parkinson's disease.Metab Brain Dis. 2025 Jan 7;40(1):90. doi: 10.1007/s11011-024-01500-x. Metab Brain Dis. 2025. PMID: 39775342 Review.
-
Pharmacological Modulators of Small GTPases of Rho Family in Neurodegenerative Diseases.Front Cell Neurosci. 2021 May 12;15:661612. doi: 10.3389/fncel.2021.661612. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34054432 Free PMC article.
-
Protective Effects of Fasudil Against Cisplatin-Induced Ototoxicity in Zebrafish: An In Vivo Study.Int J Mol Sci. 2024 Dec 13;25(24):13363. doi: 10.3390/ijms252413363. Int J Mol Sci. 2024. PMID: 39769128 Free PMC article.
-
Galangin Regulates Astrocyte Phenotypes to Ameliorate Cerebral Ischemia-reperfusion Injury by Inhibiting the RhoA/ROCK/LIMK Pathway.Curr Pharm Des. 2025;31(12):981-991. doi: 10.2174/0113816128322927241015120431. Curr Pharm Des. 2025. PMID: 39484760
-
Dasabuvir suppresses esophageal squamous cell carcinoma growth in vitro and in vivo through targeting ROCK1.Cell Death Dis. 2023 Feb 13;14(2):118. doi: 10.1038/s41419-023-05633-2. Cell Death Dis. 2023. PMID: 36781836 Free PMC article.
References
-
- Huang H, Zhu L, Reid BR, Drobny GP, Hopkins PB. Solution structure of a cisplatin-induced DNA interstrand cross-link. Science 1995;270(5243):1842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

