Porcine Reproductive and Respiratory Syndrome Virus Activates Lipophagy To Facilitate Viral Replication through Downregulation of NDRG1 Expression
- PMID: 31189711
- PMCID: PMC6694807
- DOI: 10.1128/JVI.00526-19
Porcine Reproductive and Respiratory Syndrome Virus Activates Lipophagy To Facilitate Viral Replication through Downregulation of NDRG1 Expression
Abstract
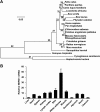
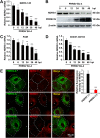
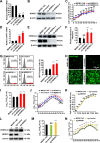
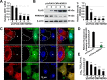
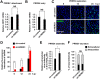
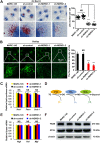
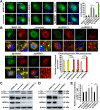
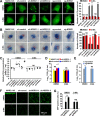
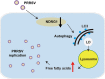
Autophagy maintains cellular homeostasis by degrading organelles, proteins, and lipids in lysosomes. Autophagy is involved in the innate and adaptive immune responses to a variety of pathogens. Some viruses can hijack host autophagy to enhance their replication. However, the role of autophagy in porcine reproductive and respiratory syndrome virus (PRRSV) infection is unclear. Here, we show that N-Myc downstream-regulated gene 1 (NDRG1) deficiency induced autophagy, which facilitated PRRSV replication by regulating lipid metabolism. NDRG1 mRNA is expressed ubiquitously in most porcine tissues and most strongly in white adipose tissue. PRRSV infection downregulated the expression of NDRG1 mRNA and protein, while NDRG1 deficiency contributed to PRRSV RNA replication and progeny virus assembly. NDRG1 deficiency reduced the number of intracellular lipid droplets (LDs), but the expression levels of key genes in lipogenesis and lipolysis were not altered. Our results also show that NDRG1 deficiency promoted autophagy and increased the subsequent yields of hydrolyzed free fatty acids (FFAs). The reduced LD numbers, increased FFA levels, and enhanced PRRSV replication were abrogated in the presence of an autophagy inhibitor. Overall, our findings suggest that NDRG1 plays a negative role in PRRSV replication by suppressing autophagy and LD degradation.IMPORTANCE Porcine reproductive and respiratory syndrome virus (PRRSV), an enveloped single-positive-stranded RNA virus, causes acute respiratory distress in piglets and reproductive failure in sows. It has led to tremendous economic losses in the swine industry worldwide since it was first documented in the late 1980s. Vaccination is currently the major strategy used to control the disease. However, conventional vaccines and other strategies do not provide satisfactory or sustainable prevention. Therefore, safe and effective strategies to control PRRSV are urgently required. The significance of our research is that we demonstrate a previously unreported relationship between PRRSV, NDRG1, and lipophagy in the context of viral infection. Furthermore, our data point to a new role for NDRG1 in autophagy and lipid metabolism. Thus, NDRG1 and lipophagy will have significant implications for understanding PRRSV pathogenesis for developing new therapeutics.
Keywords: N-Myc downstream-regulated gene 1; autophagy; lipid droplet; lipophagy; porcine reproductive and respiratory syndrome virus.
Copyright © 2019 Wang et al.
Figures
Similar articles
-
EGCG Restricts PRRSV Proliferation by Disturbing Lipid Metabolism.Microbiol Spectr. 2022 Apr 27;10(2):e0227621. doi: 10.1128/spectrum.02276-21. Epub 2022 Apr 11. Microbiol Spectr. 2022. PMID: 35404086 Free PMC article.
-
Host cells reprogram lipid droplet synthesis through YY1 to resist PRRSV infection.mBio. 2024 Aug 14;15(8):e0154924. doi: 10.1128/mbio.01549-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953350 Free PMC article.
-
Interferon-Induced Transmembrane Protein 3 Is a Virus-Associated Protein Which Suppresses Porcine Reproductive and Respiratory Syndrome Virus Replication by Blocking Viral Membrane Fusion.J Virol. 2020 Nov 23;94(24):e01350-20. doi: 10.1128/JVI.01350-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999030 Free PMC article.
-
Molecular mechanism of autophagy in porcine reproductive and respiratory syndrome virus infection.Front Cell Infect Microbiol. 2024 Aug 19;14:1434775. doi: 10.3389/fcimb.2024.1434775. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39224702 Free PMC article. Review.
-
The dual role of autophagy during porcine reproductive and respiratory syndrome virus infection: A review.Int J Biol Macromol. 2024 Dec;282(Pt 3):136978. doi: 10.1016/j.ijbiomac.2024.136978. Epub 2024 Oct 28. Int J Biol Macromol. 2024. PMID: 39471930 Review.
Cited by
-
Cellular Lipids-Hijacked Victims of Viruses.Viruses. 2022 Aug 27;14(9):1896. doi: 10.3390/v14091896. Viruses. 2022. PMID: 36146703 Free PMC article. Review.
-
EGCG Restricts PRRSV Proliferation by Disturbing Lipid Metabolism.Microbiol Spectr. 2022 Apr 27;10(2):e0227621. doi: 10.1128/spectrum.02276-21. Epub 2022 Apr 11. Microbiol Spectr. 2022. PMID: 35404086 Free PMC article.
-
Porcine reproductive and respiratory syndrome virus upregulates SMPDL3B to promote viral replication by modulating lipid metabolism.iScience. 2023 Jul 22;26(8):107450. doi: 10.1016/j.isci.2023.107450. eCollection 2023 Aug 18. iScience. 2023. PMID: 37583552 Free PMC article.
-
Early Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Infection Induces Necroptosis in Immune Cells of Peripheral Lymphoid Organs.Viruses. 2025 Feb 20;17(3):290. doi: 10.3390/v17030290. Viruses. 2025. PMID: 40143222 Free PMC article.
-
NDRG1 and its family members: More than just metastasis suppressor proteins and targets of thiosemicarbazones.J Biol Chem. 2025 Jul;301(7):110230. doi: 10.1016/j.jbc.2025.110230. Epub 2025 May 14. J Biol Chem. 2025. PMID: 40378957 Free PMC article. Review.
References
-
- Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, Liu D, Zhang S, Deng X, Ding Y, Yang L, Zhang Y, Xiao H, Qiao M, Wang B, Hou L, Wang X, Yang X, Kang L, Sun M, Jin P, Wang S, Kitamura Y, Yan J, Gao GF. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2:e526. doi:10.1371/journal.pone.0000526. - DOI - PMC - PubMed
-
- Lager KM, Schlink SN, Brockmeier SL, Miller LC, Henningson JN, Kappes MA, Kehrli ME, Loving CL, Guo B, Swenson SL, Yang HC, Faaberg KS. 2014. Efficacy of type 2 PRRSV vaccine against Chinese and Vietnamese HP-PRRSV challenge in pigs. Vaccine 32:6457–6462. doi:10.1016/j.vaccine.2014.09.046. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

