Ets-1 deficiency alleviates nonalcoholic steatohepatitis via weakening TGF-β1 signaling-mediated hepatocyte apoptosis
- PMID: 31189885
- PMCID: PMC6561928
- DOI: 10.1038/s41419-019-1672-4
Ets-1 deficiency alleviates nonalcoholic steatohepatitis via weakening TGF-β1 signaling-mediated hepatocyte apoptosis
Abstract
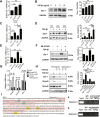
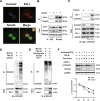
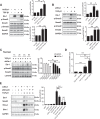
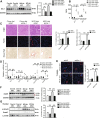
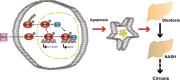
Hepatocyte apoptosis is a hallmark of nonalcoholic steatohepatitis (NASH) and contributes to liver injury, fibrosis, and inflammation. However, the molecular mechanisms underlying excessive hepatocyte apoptosis in NASH remain largely unknown. This study aimed to explore whether and how the v-ets avian erythroblastosis virus E26 oncogene homolog 1 (Ets-1) is involved in diet-induced hepatocyte apoptosis in mice. The study found that the expression level of hepatic Ets-1 was elevated in a NASH mouse model as a result of the activation of transforming growth factor beta1 (TGF-β1) signaling. In the presence of TGF-β1, phosphorylated mothers against decapentaplegic homolog 2/3 (p-Smad2/3) translocated to the binding sites of the Ets-1 promoter to upregulate the expression of Ets-1 in primary hepatocytes. In addition, Ets-1 bound directly to phosphorylated Smad3 (p-Smad3), thereby preventing the ubiquitination and proteasomal degradation of p-Smad3 and enhancing the activity of TGF-β1/Smad3 signaling. Consequently, elevated Ets-1 stimulated TGF-β1-induced hepatocyte apoptosis. However, Ets-1 knockdown alleviated diet-induced hepatocyte apoptosis and NASH with reduced liver injury, inflammation, and fibrosis. Taken together, Ets-1 had an adverse impact on hepatocyte survival under TGF-β1 treatment and accelerated the development of NASH in mice.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

