Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity
- PMID: 31189915
- PMCID: PMC6561913
- DOI: 10.1038/s41467-019-10296-9
Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity
Abstract
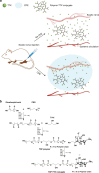
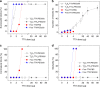
There is clinical and scientific interest in developing local anesthetics with prolonged durations of effect from single injections. The need for such is highlighted by the current opioid epidemic. Site 1 sodium channel blockers such as tetrodotoxin (TTX) are extremely potent, and can provide very long nerve blocks but the duration is limited by the associated systemic toxicity. Here we report a system where slow release of TTX conjugated to a biocompatible and biodegradable polymer, poly(triol dicarboxylic acid)-co-poly(ethylene glycol) (TDP), is achieved by hydrolysis of ester linkages. Nerve block by the released TTX is enhanced by administration in a carrier with chemical permeation enhancer (CPE) properties. TTX release can be adjusted by tuning the hydrophilicity of the TDP polymer backbone. In vivo, 1.0-80.0 µg of TTX released from these polymers produced a range of durations of nerve block, from several hours to 3 days, with minimal systemic or local toxicity.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Tetrodotoxin, Epinephrine, and Chemical Permeation Enhancer Combinations in Peripheral Nerve Blockade.Anesth Analg. 2017 Jun;124(6):1804-1812. doi: 10.1213/ANE.0000000000002072. Anesth Analg. 2017. PMID: 28452816 Free PMC article.
-
Hollow Silica Nanoparticles Penetrate the Peripheral Nerve and Enhance the Nerve Blockade from Tetrodotoxin.Nano Lett. 2018 Jan 10;18(1):32-37. doi: 10.1021/acs.nanolett.7b02461. Epub 2017 Dec 11. Nano Lett. 2018. PMID: 29227106
-
The Duration of Nerve Block from Local Anesthetic Formulations in Male and Female Rats.Pharm Res. 2019 Nov 8;36(12):179. doi: 10.1007/s11095-019-2715-3. Pharm Res. 2019. PMID: 31705417
-
Pharmacologic Properties of Novel Local Anesthetic Agents in Anesthesia Practice.Anesthesiol Clin. 2017 Jun;35(2):315-325. doi: 10.1016/j.anclin.2017.01.019. Epub 2017 Apr 14. Anesthesiol Clin. 2017. PMID: 28526152 Review.
-
Addressing the Issue of Tetrodotoxin Targeting.Mar Drugs. 2018 Sep 26;16(10):352. doi: 10.3390/md16100352. Mar Drugs. 2018. PMID: 30261623 Free PMC article. Review.
Cited by
-
Tetrodotoxin and the state-of-the-art progress of its associated analytical methods.Front Microbiol. 2024 Sep 3;15:1413741. doi: 10.3389/fmicb.2024.1413741. eCollection 2024. Front Microbiol. 2024. PMID: 39290516 Free PMC article. Review.
-
Novel Local Anesthetics in Clinical Practice: Pharmacologic Considerations and Potential Roles for the Future.Anesth Pain Med. 2022 Feb 14;12(1):e123112. doi: 10.5812/aapm.123112. eCollection 2022 Feb. Anesth Pain Med. 2022. PMID: 35433373 Free PMC article. Review.
-
Current Trends and New Challenges in Marine Phycotoxins.Mar Drugs. 2022 Mar 8;20(3):198. doi: 10.3390/md20030198. Mar Drugs. 2022. PMID: 35323497 Free PMC article. Review.
-
Novel Curcumin Derivative-Decorated Ultralong-Circulating Paclitaxel Nanoparticles: A Novel Delivery System with Superior Anticancer Efficacy and Safety.Int J Nanomedicine. 2022 Nov 14;17:5265-5286. doi: 10.2147/IJN.S369761. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36406640 Free PMC article.
-
An Updated Review of Tetrodotoxin and Its Peculiarities.Mar Drugs. 2022 Jan 3;20(1):47. doi: 10.3390/md20010047. Mar Drugs. 2022. PMID: 35049902 Free PMC article. Review.
References
-
- Pere P, Watanabe H, PitkäNen M, WahlströM T, Rosenberg PH. Local myotoxicity of bupivacaine in rabbits after continuous supraclavicular brachial plexus block. Reg. Anesth. Pain Med. 1993;18:304–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

