"Dual Disease" TgAD/GSS mice exhibit enhanced Alzheimer's disease pathology and reveal PrPC-dependent secretion of Aβ
- PMID: 31189938
- PMCID: PMC6562043
- DOI: 10.1038/s41598-019-44317-w
"Dual Disease" TgAD/GSS mice exhibit enhanced Alzheimer's disease pathology and reveal PrPC-dependent secretion of Aβ
Abstract
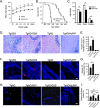
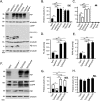
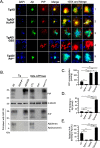
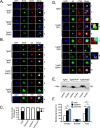
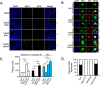
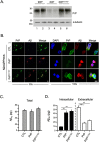
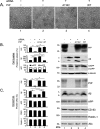
To address the question of cross-talk between prion protein (PrP) and Alzheimer's disease (AD), we generated TgAD/GSS mice that develop amyloid-β (Aβ) plaques of AD and PrP (specifically mutated PrPA116V) plaques of Gerstmann-Sträussler-Scheinker disease (GSS) and compared plaque-related features in these mice to AD mice that express normal (TgAD), high (TgAD/HuPrP), or no (TgAD/PrP-/-) PrPC. In contrast to PrPC, PrPA116V weakly co-localized to Aβ plaques, did not co-immunoprecipitate with Aβ, and poorly bound to Aβ in an ELISA-based binding assay. Despite the reduced association of PrPA116V with Aβ, TgAD/GSS and TgAD/HuPrP mice that express comparable levels of PrPA116V and PrPC respectively, displayed similar increases in Aβ plaque burden and steady state levels of Aβ and its precursor APP compared with TgAD mice. Our Tg mouse lines also revealed a predominance of intracellular Aβ plaques in mice lacking PrPC (TgAD/PrP-/-, TgAD/GSS) compared with an extracellular predominance in PrPC-expressing mice (TgAD, TgAD/HuPrP). Parallel studies in N2aAPPswe cells revealed a direct dependence on PrPC but not PrPA116V for exosome-related secretion of Aβ. Overall, our findings are two-fold; they suggest that PrP expression augments Aβ plaque production, at least in part by an indirect mechanism, perhaps by increasing steady state levels of APP, while they also provide support for a fundamental role of PrPC to bind to and deliver intraneuronal Aβ to exosomes for secretion.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
A New Transgenic Mouse Model of Gerstmann-Straussler-Scheinker Syndrome Caused by the A117V Mutation of PRNP.J Neurosci. 2009 Aug 12;29(32):10072-80. doi: 10.1523/JNEUROSCI.2542-09.2009. J Neurosci. 2009. PMID: 19675240 Free PMC article.
-
Rapamycin delays disease onset and prevents PrP plaque deposition in a mouse model of Gerstmann-Sträussler-Scheinker disease.J Neurosci. 2012 Sep 5;32(36):12396-405. doi: 10.1523/JNEUROSCI.6189-11.2012. J Neurosci. 2012. PMID: 22956830 Free PMC article.
-
Aβ-induced acceleration of Alzheimer-related τ-pathology spreading and its association with prion protein.Acta Neuropathol. 2019 Dec;138(6):913-941. doi: 10.1007/s00401-019-02053-5. Epub 2019 Aug 14. Acta Neuropathol. 2019. PMID: 31414210
-
The pathogenesis of soluble PrP fragments containing Aβ binding sites.Virus Res. 2016 Jan 4;211:194-8. doi: 10.1016/j.virusres.2015.10.023. Epub 2015 Oct 31. Virus Res. 2016. PMID: 26528810 Review.
-
How an Infection of Sheep Revealed Prion Mechanisms in Alzheimer's Disease and Other Neurodegenerative Disorders.Int J Mol Sci. 2021 May 4;22(9):4861. doi: 10.3390/ijms22094861. Int J Mol Sci. 2021. PMID: 34064393 Free PMC article. Review.
Cited by
-
The Potential Roles of Exosomes Carrying APP and Tau Cleavage Products in Alzheimer's Disease.J Clin Med. 2023 Feb 27;12(5):1883. doi: 10.3390/jcm12051883. J Clin Med. 2023. PMID: 36902671 Free PMC article. Review.
-
Proteomic signatures of corona and herpes viral antibodies identify IGDCC4 as a mediator of neurodegeneration.Sci Adv. 2025 May 30;11(22):eadt7176. doi: 10.1126/sciadv.adt7176. Epub 2025 May 30. Sci Adv. 2025. PMID: 40446030 Free PMC article.
-
β-Cleavage of the prion protein in the human eye: Implications for the spread of infectious prions and human ocular disorders.Exp Eye Res. 2021 Nov;212:108787. doi: 10.1016/j.exer.2021.108787. Epub 2021 Oct 7. Exp Eye Res. 2021. PMID: 34624335 Free PMC article.
-
Cellular prion protein acts as mediator of amyloid beta uptake by caveolin-1 causing cellular dysfunctions in vitro and in vivo.Alzheimers Dement. 2024 Oct;20(10):6776-6792. doi: 10.1002/alz.14120. Epub 2024 Aug 30. Alzheimers Dement. 2024. PMID: 39212313 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

