Strigolactones and their crosstalk with other phytohormones
- PMID: 31190074
- PMCID: PMC6868373
- DOI: 10.1093/aob/mcz100
Strigolactones and their crosstalk with other phytohormones
Abstract
Background: Strigolactones (SLs) are a diverse class of butenolide-bearing phytohormones derived from the catabolism of carotenoids. They are associated with an increasing number of emerging regulatory roles in plant growth and development, including seed germination, root and shoot architecture patterning, nutrient acquisition, symbiotic and parasitic interactions, as well as mediation of plant responses to abiotic and biotic cues.
Scope: Here, we provide a concise overview of SL biosynthesis, signal transduction pathways and SL-mediated plant responses with a detailed discourse on the crosstalk(s) that exist between SLs/components of SL signalling and other phytohormones such as auxins, cytokinins, gibberellins, abscisic acid, ethylene, jasmonates and salicylic acid.
Conclusion: SLs elicit their control on physiological and morphological processes via a direct or indirect influence on the activities of other hormones and/or integrants of signalling cascades of other growth regulators. These, among many others, include modulation of hormone content, transport and distribution within plant tissues, interference with or complete dependence on downstream signal components of other phytohormones, as well as acting synergistically or antagonistically with other hormones to elicit plant responses. Although much has been done to evince the effects of SL interactions with other hormones at the cell and whole plant levels, research attention must be channelled towards elucidating the precise molecular events that underlie these processes. More especially in the case of abscisic acid, cytokinins, gibberellin, jasmonates and salicylic acid for which very little has been reported about their hormonal crosstalk with SLs.
Keywords: Abscisic acid; D3/MAX2; D53/SMXL; GR24; auxin; cytokinins; ethylene; gibberellins; jasmonates; salicylic acid; strigolactone interactions; strigolactone signalling.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
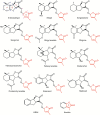




 , stimulatory effect/regulation;
, stimulatory effect/regulation;  , inhibitory effect/regulation.
, inhibitory effect/regulation.
 , stimulatory effect/regulation;
, stimulatory effect/regulation;  , possible stimulatory effect;
, possible stimulatory effect;  , inhibitory effect/regulation.
, inhibitory effect/regulation.Similar articles
-
Strigolactone and karrikin receptors regulate phytohormone biosynthetic and catabolic processes.Plant Cell Rep. 2025 Feb 21;44(3):60. doi: 10.1007/s00299-025-03456-3. Plant Cell Rep. 2025. PMID: 39982558
-
Comparing and Contrasting the Multiple Roles of Butenolide Plant Growth Regulators: Strigolactones and Karrikins in Plant Development and Adaptation to Abiotic Stresses.Int J Mol Sci. 2019 Dec 12;20(24):6270. doi: 10.3390/ijms20246270. Int J Mol Sci. 2019. PMID: 31842355 Free PMC article. Review.
-
Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research.Plant Cell Environ. 2018 Oct;41(10):2227-2243. doi: 10.1111/pce.13364. Epub 2018 Jul 24. Plant Cell Environ. 2018. PMID: 29869792 Review.
-
Phytohormone crosstalk research: cytokinin and its crosstalk with other phytohormones.Curr Protein Pept Sci. 2015;16(5):395-405. doi: 10.2174/1389203716666150330141159. Curr Protein Pept Sci. 2015. PMID: 25824387 Review.
-
Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses.Planta. 2021 Jul 9;254(2):28. doi: 10.1007/s00425-021-03678-1. Planta. 2021. PMID: 34241703 Review.
Cited by
-
The Complex Interplay between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Sinergies, Applications and Future Directions.Int J Mol Sci. 2023 Nov 26;24(23):16774. doi: 10.3390/ijms242316774. Int J Mol Sci. 2023. PMID: 38069097 Free PMC article. Review.
-
LATERAL BRANCHING OXIDOREDUCTASE, one novel target gene of Squamosa Promoter Binding Protein-like 2, regulates tillering in switchgrass.New Phytol. 2022 Jul;235(2):563-575. doi: 10.1111/nph.18140. Epub 2022 Apr 25. New Phytol. 2022. PMID: 35383390 Free PMC article.
-
Tomato Mutants Reveal Root and Shoot Strigolactone Involvement in Branching and Broomrape Resistance.Plants (Basel). 2024 Jun 4;13(11):1554. doi: 10.3390/plants13111554. Plants (Basel). 2024. PMID: 38891362 Free PMC article.
-
Crosstalk among plant hormone regulates the root development.Plant Signal Behav. 2024 Dec 31;19(1):2404807. doi: 10.1080/15592324.2024.2404807. Epub 2024 Sep 16. Plant Signal Behav. 2024. PMID: 39279500 Free PMC article. Review.
-
Strigolactone and karrikin receptors regulate phytohormone biosynthetic and catabolic processes.Plant Cell Rep. 2025 Feb 21;44(3):60. doi: 10.1007/s00299-025-03456-3. Plant Cell Rep. 2025. PMID: 39982558
References
-
- Akiyama K, Matsuzaki K, Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

