Combustible Cigarette and Smokeless Tobacco Product Preparations Differentially Regulate Intracellular Calcium Mobilization in HL60 Cells
- PMID: 31190105
- PMCID: PMC6719334
- DOI: 10.1007/s10753-019-01025-x
Combustible Cigarette and Smokeless Tobacco Product Preparations Differentially Regulate Intracellular Calcium Mobilization in HL60 Cells
Abstract
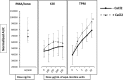
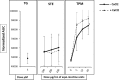
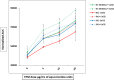
Changes in the level of intracellular calcium ([Ca2+]i) are central to leukocyte signaling and immune response. Although evidence suggests that cigarette smoking affects inflammatory response via an increase in intracellular calcium, it remains unclear if the use of smokeless tobacco (e.g., moist snuff) elicits a similar response. In this study, we evaluated the effects of tobacco product preparations (TPPs), including total particulate matter (TPM) from 3R4F reference cigarettes, smokeless tobacco extract (STE) from 2S3 reference moist snuff, and nicotine alone on Ca2+ mobilization in HL60 cells. Treatment with TPM, but not STE or nicotine alone, significantly increased [Ca2+]i in a concentration-dependent manner in HL60 cells. Moreover, TPM-induced [Ca2+]i increase was not related to extracellular Ca2+ and did not require the activation of the IP3 pathway nor involved the transient receptor potential (TRP) channels. Our findings indicate that, in cells having either intact or depleted endoplasmic reticulum (ER) Ca2+ stores, TPM-mediated [Ca2+]i increase involves cytosolic Ca2+ pools other than thapsigargin-sensitive ER Ca2+ stores. These results, for the first time, demonstrate that TPM triggers [Ca2+]i increases, while significantly higher nicotine equivalent doses of STE or nicotine alone, did not affect [Ca2+]i under the experimental conditions. In summary, our study suggests that in contrast with STE or nicotine preparations, TPM activates Ca2+ signaling pathways in HL60 cells. The differential effect of combustible and non-combustible TPPs on Ca2+ mobilization could be a useful in vitro endpoint for tobacco product evaluation.
Keywords: calcium mobilization; combustible; non-combustible; tobacco product preparations.
Conflict of interest statement
Patrudu Makena and G.L. Prasad are full-time employees of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is a wholly owned subsidiary of British American Tobacco plc. The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Evaluation of cytotoxicity of different tobacco product preparations.Regul Toxicol Pharmacol. 2012 Dec;64(3):350-60. doi: 10.1016/j.yrtph.2012.09.004. Epub 2012 Sep 17. Regul Toxicol Pharmacol. 2012. PMID: 22996032
-
Combustible and non-combustible tobacco product preparations differentially regulate human peripheral blood mononuclear cell functions.Toxicol In Vitro. 2013 Sep;27(6):1992-2004. doi: 10.1016/j.tiv.2013.06.015. Epub 2013 Jul 11. Toxicol In Vitro. 2013. PMID: 23851003
-
Cigarette and ENDS preparations differentially regulate ion channels and mucociliary clearance in primary normal human bronchial 3D cultures.Am J Physiol Lung Cell Mol Physiol. 2019 Aug 1;317(2):L295-L302. doi: 10.1152/ajplung.00096.2019. Epub 2019 Jun 5. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31166129
-
How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes.Tob Control. 2017 Mar;26(e1):e49-e58. doi: 10.1136/tobaccocontrol-2016-053060. Epub 2016 Sep 13. Tob Control. 2017. PMID: 27625408 Review.
-
Potential effects of using non-combustible tobacco and nicotine products during pregnancy: a systematic review.Harm Reduct J. 2020 Mar 2;17(1):16. doi: 10.1186/s12954-020-00359-2. Harm Reduct J. 2020. PMID: 32122384 Free PMC article.
Cited by
-
TRP Channels as Cellular Targets of Particulate Matter.Int J Mol Sci. 2021 Mar 9;22(5):2783. doi: 10.3390/ijms22052783. Int J Mol Sci. 2021. PMID: 33803491 Free PMC article. Review.
-
Cytotoxic effects of the cigarette smoke extract of heated tobacco products on human oral squamous cell carcinoma: the role of reactive oxygen species and CaMKK2.J Physiol Sci. 2024 Jun 25;74(1):35. doi: 10.1186/s12576-024-00928-1. J Physiol Sci. 2024. PMID: 38918702 Free PMC article.
-
Calcium-Dependent Pulmonary Inflammation and Pharmacological Interventions and Mediators.Biology (Basel). 2021 Oct 16;10(10):1053. doi: 10.3390/biology10101053. Biology (Basel). 2021. PMID: 34681152 Free PMC article. Review.
References
-
- Burney P, Kato B, Janson C, Mannino D, Studnicka M, Tan W, Bateman E, Koçabas A, Vollmer WM, Gislason T, Marks G, Koul PA, Gnatiuc L, Buist S, Burden of Obstructive Lung Disease (BOLD) Study Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty: a BOLD analysis—authors’ reply. Thorax. 2014;69(9):869–870. doi: 10.1136/thoraxjnl-2014-205474. - DOI - PubMed
-
- Keto J, Ventola H, Jokelainen J, Linden K, Keinänen-Kiukaanniemi S, Timonen M, Ylisaukko-oja T, Auvinen J. Cardiovascular disease risk factors in relation to smoking behaviour and history: a population-based cohort study. Open Heart. 2016;3(2):e000358. doi: 10.1136/openhrt-2015-000358. - DOI - PMC - PubMed
-
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . The health consequences of smoking-50 years of progress: a report of the surgeon general. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

