Aquaporin 4 Suppresses Neural Hyperactivity and Synaptic Fatigue and Fine-Tunes Neurotransmission to Regulate Visual Function in the Mouse Retina
- PMID: 31190144
- PMCID: PMC6834759
- DOI: 10.1007/s12035-019-01661-2
Aquaporin 4 Suppresses Neural Hyperactivity and Synaptic Fatigue and Fine-Tunes Neurotransmission to Regulate Visual Function in the Mouse Retina
Abstract
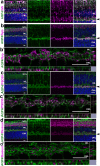
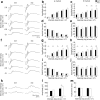
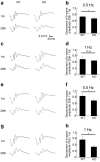
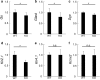
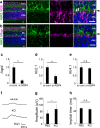
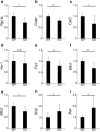
The bidirectional water channel aquaporin 4 (AQP4) is abundantly expressed in the neural tissue. The advantages and disadvantages of AQP4 neural tissue deficiency under pathological conditions, such as inflammation, and relationship with neural diseases, such as Alzheimer's disease, have been previously reported. However, the physiological functions of AQP4 are not fully understood. Here, we evaluated the role of AQP4 in the mouse retina using Aqp4 knockout (KO) mice. Aqp4 was expressed in Müller glial cells surrounding the synaptic area between photoreceptors and bipolar cells. Both scotopic and photopic electroretinograms showed hyperactive visual responses in KO mice, gradually progressing with age. Moreover, the amplitude reduction after frequent stimuli and synaptic fatigue was more severe in KO mice. Glutamine synthetase, glutamate aspartate transporter, synaptophysin, and the inward potassium channel Kir2.1, but not Kir4.1, were downregulated in KO retinas. KIR2.1 colocalized with AQP4 in Müller glial cells at the synaptic area, and its expression was affected by Aqp4 levels in primary Müller glial cell cultures. Intraocular injection of potassium in wild-type mice led to visual function hyperactivity, as observed in Aqp4 KO mice. Mitochondria molecules, such as Pgc1α and CoxIV, were downregulated, while apoptotic markers were upregulated in KO retinas. AQP4 may fine-tune synaptic activity, most likely by regulating potassium metabolism, at least in part, via collaborating with KIR2.1, and possibly indirectly regulating glutamate kinetics, to inhibit neural hyperactivity and synaptic fatigue which finally affect mitochondria and cause neurodegeneration.
Keywords: Aquaporin 4; Glutamate; Neural hyperactivity; Potassium; Retina; Synaptic fatigue.
Figures
Similar articles
-
A developmental switch in the expression of aquaporin-4 and Kir4.1 from horizontal to Müller cells in mouse retina.Invest Ophthalmol Vis Sci. 2005 Oct;46(10):3869-75. doi: 10.1167/iovs.05-0385. Invest Ophthalmol Vis Sci. 2005. PMID: 16186376
-
Reticulon 4A/Nogo-A influences the distribution of Kir4.1 but is not essential for potassium conductance in retinal Müller glia.Neurosci Lett. 2016 Aug 3;627:168-77. doi: 10.1016/j.neulet.2016.06.010. Epub 2016 Jun 6. Neurosci Lett. 2016. PMID: 27276652
-
AQP4- and Kir4.1-Mediated Müller Cell Oedema Is Involved in Retinal Injury Induced By Hypobaric Hypoxia.Mol Neurobiol. 2025 Feb;62(2):2012-2022. doi: 10.1007/s12035-024-04382-3. Epub 2024 Jul 26. Mol Neurobiol. 2025. PMID: 39060906
-
Aquaporin-4 water channels and synaptic plasticity in the hippocampus.Neurochem Int. 2013 Dec;63(7):702-11. doi: 10.1016/j.neuint.2013.05.003. Epub 2013 May 15. Neurochem Int. 2013. PMID: 23684954 Free PMC article. Review.
-
A role for aquaporin-4 in fluid regulation in the inner retina.Vis Neurosci. 2009 Mar-Apr;26(2):159-65. doi: 10.1017/S0952523809090038. Epub 2009 Apr 14. Vis Neurosci. 2009. PMID: 19366470 Review.
Cited by
-
Uveitic glaucoma-like features in Yap conditional knockout mice.Cell Death Discov. 2024 Jan 25;10(1):48. doi: 10.1038/s41420-023-01791-6. Cell Death Discov. 2024. PMID: 38272861 Free PMC article.
-
From Nature to Treatment: The Impact of Pterostilbene on Mitigating Retinal Ischemia-Reperfusion Damage by Reducing Oxidative Stress, Inflammation, and Apoptosis.Life (Basel). 2024 Sep 11;14(9):1148. doi: 10.3390/life14091148. Life (Basel). 2024. PMID: 39337931 Free PMC article.
-
Neuroprotective Effect of 4-Phenylbutyric Acid against Photo-Stress in the Retina.Antioxidants (Basel). 2021 Jul 20;10(7):1147. doi: 10.3390/antiox10071147. Antioxidants (Basel). 2021. PMID: 34356380 Free PMC article.
-
Destabilizing COXIV in Müller Glia Increases Retinal Glycolysis and Alters Scotopic Electroretinogram.Cells. 2022 Nov 24;11(23):3756. doi: 10.3390/cells11233756. Cells. 2022. PMID: 36497016 Free PMC article.
-
Effects of Epigenetic Modification of PGC-1α by a Chemical Chaperon on Mitochondria Biogenesis and Visual Function in Retinitis Pigmentosa.Cells. 2022 Apr 29;11(9):1497. doi: 10.3390/cells11091497. Cells. 2022. PMID: 35563803 Free PMC article.
References
-
- Watanabe-Matsumoto S, Moriwaki Y, Okuda T, Ohara S, Yamanaka K, Abe Y, Yasui M, Misawa H. Dissociation of blood-brain barrier disruption and disease manifestation in an aquaporin-4-deficient mouse model of amyotrophic lateral sclerosis. Neurosci Res. 2018;133:48–57. doi: 10.1016/j.neures.2017.11.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

