Efficacy of Androgen Deprivation Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Docetaxel-Based Chemotherapy
- PMID: 31190487
- PMCID: PMC7076308
- DOI: 10.5534/wjmh.190029
Efficacy of Androgen Deprivation Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Docetaxel-Based Chemotherapy
Abstract
Purpose: The purpose of this study was to determine the comparative effectiveness of androgen deprivation therapy (ADT) combined with docetaxel (DTX)-based chemotherapy in Korean and Japanese castration-resistant prostate cancer (CRPC) patient cohorts.
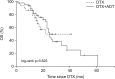
Materials and methods: Metastatic CRPC patients who underwent more than three DTX-based chemotherapy cycles in Korea and Japan between 2002 and 2017 were retrospectively analyzed and divided into the DTX-only (DTX, n=30) and combination (DTX+ADT, n=46) groups. Progression-free survival (PFS) was calculated as the time from the start of chemotherapy to the occurrence of either disease progression (prostate-specific antigen [PSA] progression or radiographic progression) or death. The primary end point was PFS and the secondary end point was overall survival (OS).
Results: In the DTX and DTX+ADT groups, the median PFS was 6.0 and 11.0 months (log-rank p=0.053). The multivariate Cox regression analysis revealed that the significant predicting factors of PFS were ADT administration (hazard ratio [HR], 0.478; 95% confidence interval [CI], 0.284-0.804; p=0.005) and number of DTX-based chemotherapy cycles (HR, 0.934; 95% CI, 0.899-0.970; p<0.001). In the DTX and DTX+ADT groups, the median OS was 16.0 and 19.5 months (log-rank p=0.825). Through multiple Cox regression analysis, we found that the significant predicting factors of OS were the PSA nadir level (HR, 1.001; 95% CI, 1.000-1.002; p<0.001) and number of DTX-based chemotherapy cycles (HR, 0.932; 95% CI, 0.876-0.991; p=0.024).
Conclusions: Concurrent DTX-based chemotherapy and ADT may be beneficial compared with DTX-based chemotherapy alone in chemotherapy-naïve metastatic CRPC patients in terms of the PFS, but not the OS.
Keywords: Antineoplastic hormonal drugs; Docetaxel; Progression-free survival; Prostate cancer.
Copyright © 2020 Korean Society for Sexual Medicine and Andrology.
Conflict of interest statement
The authors have nothing to disclose.
Figures

Similar articles
-
Survival Outcomes of Concurrent Treatment with Docetaxel and Androgen Deprivation Therapy in Metastatic Castration-Resistant Prostate Cancer.Yonsei Med J. 2016 Sep;57(5):1070-8. doi: 10.3349/ymj.2016.57.5.1070. Yonsei Med J. 2016. PMID: 27401636 Free PMC article.
-
PSA nadir and time to PSA nadir during initial androgen deprivation therapy as prognostic factors in metastatic castration-resistance prostate cancer patients treated with docetaxel.Andrologia. 2021 May;53(4):e13916. doi: 10.1111/and.13916. Epub 2021 Feb 16. Andrologia. 2021. PMID: 33591598
-
[Predictive factor analysis of time to progression of castration-resistant prostate cancer after androgen deprivation therapy].Beijing Da Xue Xue Bao Yi Xue Ban. 2017 Aug 18;49(4):657-662. Beijing Da Xue Xue Bao Yi Xue Ban. 2017. PMID: 28816284 Chinese.
-
Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Castration-resistant Prostate Cancer with Next generation Androgen Receptor Signal Inhibition: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Jul;4(4):529-539. doi: 10.1016/j.euf.2017.01.004. Epub 2017 Jan 23. Eur Urol Focus. 2018. PMID: 28753843
-
Anemia is associated with poor outcomes of metastatic castration-resistant prostate cancer, a systematic review and meta-analysis.Am J Transl Res. 2018 Dec 15;10(12):3877-3886. eCollection 2018. Am J Transl Res. 2018. PMID: 30662637 Free PMC article. Review.
Cited by
-
Docetaxel Enhances Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Mediated Apoptosis in Prostate Cancer Cells via Epigenetic Gene Regulation by Enhancer of Zeste Homolog 2.World J Mens Health. 2023 Jul;41(3):649-658. doi: 10.5534/wjmh.220073. Epub 2023 Jan 1. World J Mens Health. 2023. PMID: 36593705 Free PMC article.
-
Maintenance of androgen deprivation therapy or testosterone supplementation in the management of castration-resistant prostate cancer: that is the question.Endocrine. 2022 Dec;78(3):441-445. doi: 10.1007/s12020-022-03166-w. Epub 2022 Aug 20. Endocrine. 2022. PMID: 35986139 Free PMC article.
-
Lignans: Advances in Biosynthesis, Bioavailability, and Pharmacological Activity.Comb Chem High Throughput Screen. 2025;28(8):1331-1344. doi: 10.2174/0113862073316439240515072946. Comb Chem High Throughput Screen. 2025. PMID: 38779732 Review.
-
Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites.Int J Mol Sci. 2022 Dec 7;23(24):15482. doi: 10.3390/ijms232415482. Int J Mol Sci. 2022. PMID: 36555124 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol. 2018;25:524–531. - PubMed
-
- Kakehi Y, Sugimoto M, Taoka R Committee for Establishment of the evidenced-based clinical practice guideline for prostate cancer of the Japanese Urological Association. Evidenced-based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition) Int J Urol. 2017;24:648–666. - PubMed
-
- Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

