Efficacy of Androgen Deprivation Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Docetaxel-Based Chemotherapy
- PMID: 31190487
- PMCID: PMC7076308
- DOI: 10.5534/wjmh.190029
Efficacy of Androgen Deprivation Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Docetaxel-Based Chemotherapy
Abstract
Purpose: The purpose of this study was to determine the comparative effectiveness of androgen deprivation therapy (ADT) combined with docetaxel (DTX)-based chemotherapy in Korean and Japanese castration-resistant prostate cancer (CRPC) patient cohorts.
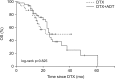
Materials and methods: Metastatic CRPC patients who underwent more than three DTX-based chemotherapy cycles in Korea and Japan between 2002 and 2017 were retrospectively analyzed and divided into the DTX-only (DTX, n=30) and combination (DTX+ADT, n=46) groups. Progression-free survival (PFS) was calculated as the time from the start of chemotherapy to the occurrence of either disease progression (prostate-specific antigen [PSA] progression or radiographic progression) or death. The primary end point was PFS and the secondary end point was overall survival (OS).
Results: In the DTX and DTX+ADT groups, the median PFS was 6.0 and 11.0 months (log-rank p=0.053). The multivariate Cox regression analysis revealed that the significant predicting factors of PFS were ADT administration (hazard ratio [HR], 0.478; 95% confidence interval [CI], 0.284-0.804; p=0.005) and number of DTX-based chemotherapy cycles (HR, 0.934; 95% CI, 0.899-0.970; p<0.001). In the DTX and DTX+ADT groups, the median OS was 16.0 and 19.5 months (log-rank p=0.825). Through multiple Cox regression analysis, we found that the significant predicting factors of OS were the PSA nadir level (HR, 1.001; 95% CI, 1.000-1.002; p<0.001) and number of DTX-based chemotherapy cycles (HR, 0.932; 95% CI, 0.876-0.991; p=0.024).
Conclusions: Concurrent DTX-based chemotherapy and ADT may be beneficial compared with DTX-based chemotherapy alone in chemotherapy-naïve metastatic CRPC patients in terms of the PFS, but not the OS.
Keywords: Antineoplastic hormonal drugs; Docetaxel; Progression-free survival; Prostate cancer.
Copyright © 2020 Korean Society for Sexual Medicine and Andrology.
Conflict of interest statement
The authors have nothing to disclose.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol. 2018;25:524–531. - PubMed
-
- Kakehi Y, Sugimoto M, Taoka R Committee for Establishment of the evidenced-based clinical practice guideline for prostate cancer of the Japanese Urological Association. Evidenced-based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition) Int J Urol. 2017;24:648–666. - PubMed
-
- Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

