Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: evidence to date
- PMID: 31190735
- PMCID: PMC6514126
- DOI: 10.2147/DDDT.S170969
Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: evidence to date
Abstract
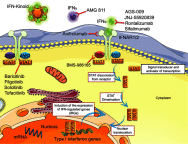
Previous reports have described the appearance of systemic lupus erythematosus (SLE) cases following interferon-α (IFN-α) therapy, IFN-regulated gene expression is significantly increased in SLE, and an association between SLE and gene variants belonging to IFN downstream pathways has been shown. Based on this, targeting of IFN and of their signaling pathways has appeared to be interesting developments within the field of SLE therapy. Different specific type I IFN antagonists have been studied in clinical trials and some of those have already reached Phase III. A potential approach would be to target IFN receptors rather than IFN themselves. Anifrolumab (previously MEDI-546) is a fully human monoclonal antibody (Ab) that binds to subunit 1 of the type I IFN receptor (IFNAR1), blocking the action of different type I IFNs (IFN-α, IFN-β and IFN-ω). This drug has been assessed in 11 clinical studies: 9 in SLE, 1 in systemic sclerosis and 1 in rheumatoid arthritis. In SLE, clinical development reached Phase I for 1 study and Phases II and III for 5 and 3 trials, respectively. The Phase IIb, randomized control trial (RCT), double-blind, placebo-controlled study of adults with moderate-to-severe SLE (MUSE trial) showed positive results on the composite primary endpoint SRI-4. Greater efficacy was seen in patients with high baseline IFN gene signature compared with those with low baseline IFN gene signature. Anifrolumab also demonstrated promising results on cutaneous and arthritic manifestations, especially among patients with a high IFN gene signature. The pivotal Treatment of Uncontrolled Lupus via the Interferon IFN Pathway (TULIP 1 and 2 studies are now completed. In August 2018, the promoter announced that the TULIP 1 Phase III trial did not reach its primary endpoint. The release of the completed but not yet published Phase II studies and of the TULIP pivotal trials results will further inform us on the actual therapeutic potential of anifrolumab.
Keywords: anifrolumab; interferon type I; interferon-alpha; receptors interferon; systemic lupus erythematosus.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Interferon Inhibition for Lupus with Anifrolumab: Critical Appraisal of the Evidence Leading to FDA Approval.ACR Open Rheumatol. 2022 Jun;4(6):486-491. doi: 10.1002/acr2.11414. Epub 2022 Feb 14. ACR Open Rheumatol. 2022. PMID: 35157371 Free PMC article.
-
Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials.Mod Rheumatol. 2021 Jan;31(1):1-12. doi: 10.1080/14397595.2020.1812201. Epub 2020 Sep 17. Mod Rheumatol. 2021. PMID: 32814461 Review.
-
Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus.Arthritis Rheumatol. 2017 Feb;69(2):376-386. doi: 10.1002/art.39962. Arthritis Rheumatol. 2017. PMID: 28130918 Free PMC article. Clinical Trial.
-
Type I interferon blockade with anifrolumab in patients with systemic lupus erythematosus modulates key immunopathological pathways in a gene expression and proteomic analysis of two phase 3 trials.Ann Rheum Dis. 2024 Jul 15;83(8):1018-1027. doi: 10.1136/ard-2023-225445. Ann Rheum Dis. 2024. PMID: 38569851 Free PMC article. Clinical Trial.
-
Anifrolumab: first biologic approved in the EU not restricted to patients with a high degree of disease activity for the treatment of moderate to severe systemic lupus erythematosus.Expert Rev Clin Immunol. 2024 Jan-Jun;20(1):21-30. doi: 10.1080/1744666X.2023.2268284. Epub 2024 Jan 8. Expert Rev Clin Immunol. 2024. PMID: 37800604 Review.
Cited by
-
Therapeutically targeting proinflammatory type I interferons in systemic lupus erythematosus: efficacy and insufficiency with a specific focus on lupus nephritis.Front Immunol. 2024 Oct 16;15:1489205. doi: 10.3389/fimmu.2024.1489205. eCollection 2024. Front Immunol. 2024. PMID: 39478861 Free PMC article. Review.
-
Interaction of antiphospholipid antibodies with endothelial cells in antiphospholipid syndrome.Front Immunol. 2024 Jul 9;15:1361519. doi: 10.3389/fimmu.2024.1361519. eCollection 2024. Front Immunol. 2024. PMID: 39044818 Free PMC article. Review.
-
Clinical Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Anifrolumab.Clin Pharmacokinet. 2023 May;62(5):655-671. doi: 10.1007/s40262-023-01238-2. Epub 2023 May 6. Clin Pharmacokinet. 2023. PMID: 37148484 Free PMC article. Review.
-
Induction of LY6E regulates interleukin-1β production, potentially contributing to the immunopathogenesis of systemic lupus erythematosus.Cell Commun Signal. 2025 Mar 20;23(1):146. doi: 10.1186/s12964-025-02140-z. Cell Commun Signal. 2025. PMID: 40114200 Free PMC article.
-
Early type I IFN blockade improves the efficacy of viral vaccines.J Exp Med. 2020 Dec 7;217(12):e20191220. doi: 10.1084/jem.20191220. J Exp Med. 2020. PMID: 32820330 Free PMC article.
References
-
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical