Collecting patient preference information using a Clinical Data Research Network: demonstrating feasibility with idiopathic pulmonary fibrosis
- PMID: 31190761
- PMCID: PMC6529600
- DOI: 10.2147/PPA.S201632
Collecting patient preference information using a Clinical Data Research Network: demonstrating feasibility with idiopathic pulmonary fibrosis
Abstract
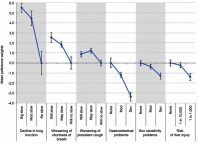
Purpose: Rare diseases present challenges for accessing patient populations to conduct surveys. Clinical Data Research Networks (CDRNs) offer an opportunity to overcome those challenges by providing infrastructure for accessing patients and sharing data. This study aims to demonstrate the feasibility of collecting patient preference information for a rare disease in a CDRN, using idiopathic pulmonary fibrosis as proof of concept. Patients and methods: Utilizing a cohort of idiopathic pulmonary fibrosis (IPF) patients across a CDRN, a discrete choice experiment was administered via electronic and paper methods to collect patient preference information about benefits and risks of two therapeutic options. Survey data were augmented with data from electronic health records and patient-reported outcome surveys. Results: Thirty-three patients completed the preference experiment. The amount of choice attributable to a benefit of slowing of decline in lung function was 36%. Improving efficacy in terms of lung function was 2.16 times as important as improving efficacy in terms of shortness of breath. In terms of side effects, decreasing risk of gastrointestinal problems was 2.6 times as important as decreasing risk of sun sensitivity and 2.4 times as important as decreasing risk of liver injury. In terms of benefit-risk trade-offs, improving efficacy in terms of lung function was 1.6 times as important as decreasing risk of gastrointestinal problems. Conclusion: This study used IPF as a proof of concept to demonstrate the feasibility of collecting patient preference information in a CDRN. The network was advantageous to the study of patient preferences. Future research should continue to explore pathways for the collection and use of patient preference information across networks. The power of consolidated collection efforts may lead to the ability to use preference data to inform decision-making at the regional, specialty, or individual encounter level.
Keywords: benefit-risk trade-off; discrete choice experiment; patient-centered outcomes research; stated preference methods.
Conflict of interest statement
Dr Ilene L Hollin reports grants from Center for Medicine in the Public Interest, during the conduct of the study and employment with the National Pharmaceutical Council (NPC), outside the submitted work. Ms Anne EF Dimmock reports holding the position of a study coordinator for clinical trials conducted by the pharmaceutical companies who manufacture both nintedanib (OFEV) and pirfenidone (Esbriet), the two medications referenced in the manuscript. Dr Sonye K Danoff reports personal fees from Boehringer Ingelheim, grants from Genentech/Roche, and personal fees from Galapagos, NV, outside the submitted work. Dr Rebecca Bascom reports Penn State College of Medicine received a research award from PCORI that funded the PaTH CDRN which conducted the IPF cohort study from which the participants for this project were recruited. Dr Bascom was clinical lead for the IPF cohort. Penn State College of Medicine has received funding from InterMune and Boehringer Ingelheim to be a study site to evaluate the antifibrotics pirfenidone and nintedanib. Dr Bascom was a site principal investigator for these studies. The authors report no other conflicts of interest in this work.
Figures
References
-
- Medical Device Innovation Consortium (MDIC). Medical Device Innovation Consortium (MDIC) Patient Centered Benefit-Risk Project Report: A Framework for Incorporating Information on Patient Preferences regarding Benefit and Risk into Regulatory Assessments of New Medical Technology Arlington, VA: 2012.
-
- US Food and Drug Administration. Patient Preference Information – Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling: Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Rockville (MD): US Department of Health and Human Services Food and Drug Administration, Center for Devices and Radiological Health, Center for Biological Evaluation and Research; 2016.
-
- US Food and Drug Administration. Patient-Focused Drug Development: Collecting Comprehensive and Representative Input: Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. Rockville (MD): US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biological Evaluation and Research; 2018.
LinkOut - more resources
Full Text Sources