Medication management patterns among Medicare beneficiaries with chronic obstructive pulmonary disease who initiate nebulized arformoterol treatment
- PMID: 31190787
- PMCID: PMC6526678
- DOI: 10.2147/COPD.S199251
Medication management patterns among Medicare beneficiaries with chronic obstructive pulmonary disease who initiate nebulized arformoterol treatment
Abstract
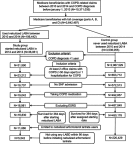
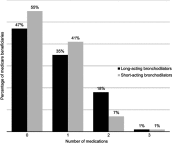
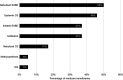
Purpose: Global evidence-based treatment strategies for chronic obstructive pulmonary disease (COPD) recommend using long-acting bronchodilators (LABDs) as maintenance therapy. However, COPD patients are often undertreated. We examined COPD treatment patterns among Medicare beneficiaries who initiated arformoterol tartrate, a nebulized long-acting beta2 agonist (LABA), and identified the predictors of initiation. Methods: Using a 100% sample of Medicare administrative data, we identified beneficiaries with a COPD diagnosis (ICD-9 490-492.xx, 494.xx, 496.xx) between 2010 and 2014 who had ≥1 year of continuous enrollment in Parts A, B, and D, and ≥2 COPD-related outpatient visits within 30 days or ≥1 hospitalization(s). After applying inclusion/exclusion criteria, three cohorts were identified: (1) study group beneficiaries who received nebulized arformoterol (n=11,886), (2) a subset of the study group with no LABD use 90 days prior to initiating arformoterol (n=5,542), and (3) control group beneficiaries with no nebulized LABA use (n=220,429). Logistic regression was used to evaluate predictors of arformoterol initiation. Odds ratios (ORs), 95% confidence intervals (CIs), and p values were computed. Results: Among arformoterol users, 47% (n=5,542) had received no LABDs 90 days prior to initiating arformoterol. These beneficiaries were being treated with a nebulized (50%) or inhaled (37%) short-acting bronchodilator or a systemic corticosteroid (46%), and many received antibiotics (37%). Compared to controls, beneficiaries who initiated arformoterol were significantly more likely to have had an exacerbation, a COPD-related hospitalization, and a pulmonologist or respiratory therapist visit prior to initiation (all p<0.05). Beneficiaries with moderate/severe psychiatric comorbidity or dual-eligible status were significantly less likely to initiate arformoterol, as compared to controls (all p<0.05). Conclusion: Medicare beneficiaries who initiated nebulized arformoterol therapy had more exacerbations and hospitalizations than controls 90 days prior to initiation. Findings revealed inadequate use of maintenance medications, suggesting a lack of compliance with evidence-based treatment guidelines.
Keywords: COPD; Medicare; arformoterol; long-acting beta2-agonists; nebulized therapy; treatment patterns.
Conflict of interest statement
BRC received consultation remuneration as a member of the Medical Advisory Board at Advance Health Solutions, LLC. He has also been an expert pulmonologist consultant for Glaxo Smith Kline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. He reports personal fees from Boehringer INgelheim, Glaxo Smith Kline, Astra Zeneca, Novartis, and Chiesi, outside the submitted work. MN and SCR are employed by Advance Health Solutions, LLC which received funding from Sunovion Pharmaceuticals Inc. to oversee this study. ZX and TPG are employed by the University of California San Diego which received a grant from Advance Health Solutions, LLC to conduct this study. CD is employed by Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
Figures

References
-
- Murphy SL, Xu JQ, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015 In: Sudol. J, editor. National Vital Statistics Reports. Vol. 66 no. 6 Hyattsville, MD: National Center for Health Statistics; 2017. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_06.pdf. Accessed December19, 2018. - PubMed
-
- Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease. [Updated June6, 2018]. Available from: https://www.cdc.gov/copd/index.html. Accessed November1, 2018.
-
- Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for the diagnosis, management, and prevention of COPD. [Updated 2018]. Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-rev.... Accessed November1, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

