Supplementation with high-content docosahexaenoic acid triglyceride in attention-deficit hyperactivity disorder: a randomized double-blind placebo-controlled trial
- PMID: 31190827
- PMCID: PMC6514260
- DOI: 10.2147/NDT.S206020
Supplementation with high-content docosahexaenoic acid triglyceride in attention-deficit hyperactivity disorder: a randomized double-blind placebo-controlled trial
Abstract
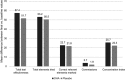
Background: Attention-deficit hyperactivity disorder (ADHD) is a complex disorder in terms of etiology, clinical presentation, and treatment outcome. Pharmacological and psychological interventions are recommended as primary treatments in ADHD; however, other nonpharmacological intervention such as a dietary supplementation with omega-3 polyunsaturated fatty acids (ω-3 PUFAs) has emerged as an attractive option. Purpose: The objective of the present study was to assess whether dietary supplementation with highly concentrated ω-3 docosahexaenoic acid (DHA) triglyceride may improve symptoms in ADHD. Method: A 6-month prospective double-blind placebo-controlled randomized clinical trial was designed in 66 patients with ADHD, aged between 6 and 18 years. Participants in the experimental group received a combination of ω-3 fatty acids (DHA 1,000 mg, eicosapentaenoic acid 90 mg, and docosapentaenoic acid 150 mg). Instruments included d2-test, AULA Nesplora, EDAH scales, and abbreviated Conner's Rating Scale. Results: In the cognitive test, between-group differences were not found, but within-group differences were of a greater magnitude in the DHA group. Between-group differences in favor of the DHA arm were observed in behavioral measures, which were already detected after 3 months of treatment. Results were not changed when adjusted by ADHD medication. Conclusions: This study provides further evidence of the beneficial effect of supplementation with ω-3 DHA in the management of ADHD.
Keywords: PUFAs; attention-deficit hyperactivity disorder (ADHD); docosahexaenoic acid (DHA); eicosapentaenoic acid (EPA); omega 3.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Catalá-López F, Hutton B, Núñez-Beltrán A, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: protocol for a systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2015;4(1):19. doi:10.1186/s13643-015-0005-7 - DOI - PMC - PubMed
-
- García T, Rodríguez C, Rodríguez J, Fernández-Suárez A, Richarte V, Ramos-Quiroga JA. Psychosocial profiles of adults with ADHD: a comparative study of prison and outpatient psychiatric samples. Eur J Psychol Appl L. 2019;11:41–49. doi:10.5093/ejpalc2018a14 - DOI

