miR-129-5p inhibits prostate cancer proliferation via targeting ETV1
- PMID: 31190859
- PMCID: PMC6512784
- DOI: 10.2147/OTT.S183435
miR-129-5p inhibits prostate cancer proliferation via targeting ETV1
Abstract
Background: Prostate cancer is one of the most commonly diagnosed diseases in males.
Methods: RT-qPCR was used to detect miR-129-5p expression in tumor tissues and adjacent normal tissues from patients with prostate cancer. The cell proliferation assay and colony forming assay were used to study the role of miR-129-5p in mediating prostate cancer cell growth. Bioinformatic analysis and dual luciferase assay were performed to predict and confirm ETV1 as a target gene of miR-129-5p.
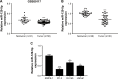
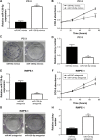
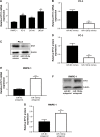
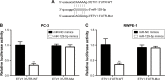
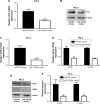
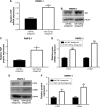
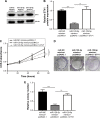
Results: We found that miR-129-5p levels were decreased significantly in human prostate cancer tissues compared with matched normal tissues from patients with prostate cancer. Overexpression of miR-129-5p suppressed prostate cancer cell growth while antagonist of miR-129-5p promoted cell proliferation in immortal prostate cell line RWPE-1. In addition, elevation of miR-129-5p decreased ETV1 expression in prostate cancer cells while downregulation of miR-129-5p increased ETV1 expression in RWPE-1. Mechanistically, ETV1 is confirmed a direct target of miR-129-5p in prostate cancer cells. Through repression of ETV1 expression, miR-129-5p could inactivate YAP signaling in prostate cancer cells. In addition, overexpression of ETV1 attenuated miR-129-5p induced cell proliferation in prostate cancer cells. Correlation analysis further revealed that there was a negative correlation between miR-129-5p levels and ETV1 mRNA levels in tumor tissues from patients with prostate cancer.
Conclusion: Our results identified miR-129-5p as a tumor suppressor in prostate cancer via repression of ETV1.
Keywords: ETV1; microRNA; proliferation; prostate cancer.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer. JAMA. 2017;317(24):2532–2542. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Antenor JA, Roehl KA, Eggener SE, Kundu SD, Han M, Catalona WJ. Preoperative PSA and progression-free survival after radical prostatectomy for stage T1c disease. Urology. 2005;66(1):156–160. - PubMed
LinkOut - more resources
Full Text Sources

