Impact of CXCR4 and CXCR7 knockout by CRISPR/Cas9 on the function of triple-negative breast cancer cells
- PMID: 31190884
- PMCID: PMC6527053
- DOI: 10.2147/OTT.S195661
Impact of CXCR4 and CXCR7 knockout by CRISPR/Cas9 on the function of triple-negative breast cancer cells
Abstract
Background: Breast cancer is one of the most common malignancies threatening women's health. Triple-negative breast cancer (TNBC) is a special type of breast cancer with high invasion and metastasis. CXCL12 and its receptors CXCR4 and CXCR7 play a crucial role in the progress of breast cancer. The aim of this study was to investigate the effect of CXCR4 and CXCR7 on the function of TNBC.
Materials and methods: We used the CRISPR/Cas9 technique to carry out a single knockout of the CXCR4 or CXCR7 gene and co-knockout of CXCR4 and CXCR7 genes in the TNBC cell line (MDA-MB-231). The single knockout and co-knockout cells were screened and verified by PCR sequencing and Western blot assay, the effect of single knockout and co-knockout on the proliferation of TNBC cells was examined using the Cell Counting Kit-8 and colony formation assays, the migration and invasion of TNBC cells were examined by the transwell and wound-healing assays, the changes in the cell cycle distribution after knockout were detected by flow cytometry, and the difference in the migration and invasion of single knockout and co-knockout induced by CXCL12 was observed by adding CXCL12 in the experimental group.
Results: The single knockout of the CXCR4 or CXCR7 gene significantly reduced the cell proliferation, growth, migration, and invasion and delayed the conversion of the G1/S cycle, while the co-knockout inhibited these biological abilities more significantly. In both the knockout and control groups, the migration and invasion of CXCL12-added cells were significantly stronger than those of the non-CXCL12-added cells, and CXCL12 induced lesser migration and invasion in the CXCR4 and CXCR7 co-knockout group than in the single knockout groups.
Conclusion: The knockout of the CXCR4 and CXCR7 genes affects the binding capacity and functions of CXCL12, inhibits the malignant progression of TNBC cells significantly, and may become a potential target for the treatment of TNBC.
Keywords: CRISPR/Cas9; CXCL12; CXCR4; CXCR7; TNBC; triple-negative breast cancer cells.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

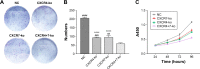
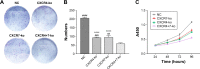


References
LinkOut - more resources
Full Text Sources
Miscellaneous

