Mitochondria P-glycoprotein confers paclitaxel resistance on ovarian cancer cells
- PMID: 31190887
- PMCID: PMC6529025
- DOI: 10.2147/OTT.S193433
Mitochondria P-glycoprotein confers paclitaxel resistance on ovarian cancer cells
Abstract
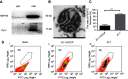
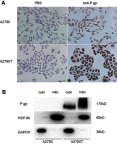
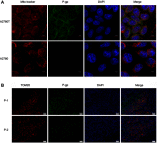
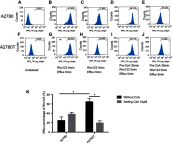
Background: Subcellular expression of P-glycoprotein (P-gp) may play an essential role in multidrug resistance (MDR) in many cancers. However, the mitochondria expression and functional activity of P-gp in ovarian cancer are still unclear. In this study, we isolated mitochondria from A2780 cell line and its paclitaxel-resistant subline A2780T and investigated the expression and function of mitochondria P-gp. Methods: Immunocytochemistry was used to evaluate P-gp expression and subcellular localization in cancer cells. Immunofluorescence and laser confocal microscopy were used to detect the co-localization of P-gp and mitochondria both in ovarian cancer tissues and in cell lines. Western blotting (WB), transmission electron microscopy and JC-1 kit were used to evaluate the purity, integrity and activity of the isolated mitochondria. P-gp expression in the whole cell and the isolated mitochondria was evaluated by WB. Flow cytometry was used to evaluate the efflux function of mitochondria P-gp. Results: P-gp expression was detected at the membrane, cytoplasm and nuclei of the A2780T cells, but not in the A2780 cells. Co-localization of P-gp and mitochondria was observed in the A2780T cell line and ovarian cancer tissues, but not in A2780 cells. The purity, integration and activity of the isolated mitochondria are high. P-gp was highly expressed in the A2780T cells and the isolated mitochondria, but was not found in A2780 cells. Rho123 efflux rate was significantly increased in isolated A2780T mitochondria compared to those in A2780 (43.2% vs 9.6%), but it was partly reversed by cyclosporin A (CsA, a P-gp inhibitor). Conclusion: P-gp is highly expressed in mitochondria of taxol-resistant ovarian cancer cells and ovarian cancer tissues and mediates the drug efflux, which probably protect cancer cells from chemotherapy.
Keywords: P-glycoprotein; mitochondria; multidrug resistance; ovarian carcinoma.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the international cancer benchmarking partnership): an analysis of population-based cancer registry data. Lancet (London, England). 2011;377(9760):127–138. doi: 10.1016/S0140-6736(10)62231-3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

