Effects of sub-inhibitory concentrations of meropenem and tigecycline on the expression of genes regulating pili, efflux pumps and virulence factors involved in biofilm formation by Acinetobacter baumannii
- PMID: 31190904
- PMCID: PMC6512781
- DOI: 10.2147/IDR.S199993
Effects of sub-inhibitory concentrations of meropenem and tigecycline on the expression of genes regulating pili, efflux pumps and virulence factors involved in biofilm formation by Acinetobacter baumannii
Abstract
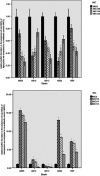
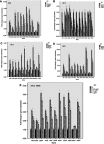
Background: Sub-minimal inhibitory concentrations of antibiotics have been indicated to affect the biofilm formation in pathogens of nosocomial infections. This study aimed to investigate the effects of meropenem and tigecycline at their sub-minimum inhibitory concentrations (MICs) on the biofilm formation capacity of Acinetobacter baumannii (A. baumannii), as well as the expression levels of genes involved in biofilm formation, quorum sensing, pili assembly and efflux pumps. Materials and methods: In this study, four non-clonal strains (AB10, AB13, AB32 and AB55), which were different from the aspects of antibiotic susceptibility and biofilm formation from each other were selected for the evaluation of antimicrobial susceptibility, biofilm inducibility at sub-MICs of meropenem and tigecycline and the gene expression levels (the abaI, abaR, bap, pgaA, csuE, bfmS, bfmR, ompA, adeB, adeJ and adeG genes). Result: A significant increase in the MICs of all antibiotics was demonstrated in the biofilm cells in each four strains. The biofilm formation was significantly decreased in all the representative strains exposed to tigecycline. However, the biofilm inducibility at sub-MICs of meropenem was dependent on strain genotype. In concordance with these results, Pearson correlation analysis indicated a positive significant correlation between the biofilm formation capacity and the mRNA levels of genes encoding efflux pumps except adeJ, the genes involved in biofilm formation, pili assembly and quorum sensing following exposure to meropenem and tigecycline at their sub-MICs. Conclusion: These results revealed valuable data into the correlation between the gene transcription levels and biofilm formation, as well as quorum sensing and their regulation at sub-MICs of meropenem and tigecycline.
Keywords: Acinetobacter baumannii; biofilm formation; gene expression; meropenem; sub-MIC; tigecycline.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Sub-minimum inhibitory concentrations of colistin and polymyxin B promote Acinetobacter baumannii biofilm formation.PLoS One. 2018 Mar 19;13(3):e0194556. doi: 10.1371/journal.pone.0194556. eCollection 2018. PLoS One. 2018. PMID: 29554105 Free PMC article.
-
Biofilm Formation Caused by Clinical Acinetobacter baumannii Isolates Is Associated with Overexpression of the AdeFGH Efflux Pump.Antimicrob Agents Chemother. 2015 Aug;59(8):4817-25. doi: 10.1128/AAC.00877-15. Epub 2015 Jun 1. Antimicrob Agents Chemother. 2015. PMID: 26033730 Free PMC article.
-
Sub-minimum inhibitory concentrations (sub-MICs) of colistin on Acinetobacter baumannii biofilm formation potency, adherence, and invasion to epithelial host cells: an experimental study in an Iranian children's referral hospital.Microbiol Spectr. 2024 Feb 6;12(2):e0252323. doi: 10.1128/spectrum.02523-23. Epub 2024 Jan 17. Microbiol Spectr. 2024. PMID: 38230925 Free PMC article.
-
Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection.Front Med (Lausanne). 2022 Mar 24;9:793615. doi: 10.3389/fmed.2022.793615. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35402433 Free PMC article. Review.
-
Environmental and clinical impacts of antibiotics' sub-minimum inhibitory concentrations on the development of resistance in acinetobacter baumannii.Sci Total Environ. 2025 Jun 1;979:179521. doi: 10.1016/j.scitotenv.2025.179521. Epub 2025 Apr 27. Sci Total Environ. 2025. PMID: 40288165 Review.
Cited by
-
In-Human Multiyear Evolution of Carbapenem-Resistant Klebsiella pneumoniae Causing Chronic Colonization and Intermittent Urinary Tract Infections: A Case Study.mSphere. 2022 Jun 29;7(3):e0019022. doi: 10.1128/msphere.00190-22. Epub 2022 May 9. mSphere. 2022. PMID: 35531657 Free PMC article.
-
Sub-inhibitory concentrations of colistin and imipenem impact the expression of biofilm-associated genes in Acinetobacter baumannii.Arch Microbiol. 2024 Mar 15;206(4):169. doi: 10.1007/s00203-024-03869-w. Arch Microbiol. 2024. PMID: 38489041
-
Association Between Biofilm Formation, Structure, and the Expression Levels of Genes Related to biofilm formation and Biofilm-Specific Resistance of Acinetobacter baumannii Strains Isolated from Burn Infection in Ahvaz, Iran.Infect Drug Resist. 2019 Dec 12;12:3867-3881. doi: 10.2147/IDR.S228981. eCollection 2019. Infect Drug Resist. 2019. PMID: 31853190 Free PMC article.
-
Different effects of sub-minimum inhibitory concentrations of gentamicin on the expression of genes involved in alginate production and biofilm formation of Pseudomonas aeruginosa.Iran J Microbiol. 2021 Dec;13(6):808-816. doi: 10.18502/ijm.v13i6.8085. Iran J Microbiol. 2021. PMID: 35222859 Free PMC article.
-
Subinhibitory Concentrations of Clinically-Relevant Antimicrobials Affect Resistance-Nodulation-Division Family Promoter Activity in Acinetobacter baumannii.Front Microbiol. 2021 Dec 3;12:780201. doi: 10.3389/fmicb.2021.780201. eCollection 2021. Front Microbiol. 2021. PMID: 34925284 Free PMC article.
References
-
- Longo F, Vuotto C, Donelli G. Biofilm formation in acinetobacter baumannii. New Microbiol. 2014;37(2):119–127. - PubMed
-
- Selasi GN, Nicholas A, Jeon H, et al. Differences in biofilm mass, expression of biofilm-associated genes, and resistance to desiccation between epidemic and sporadic clones of carbapenem-resistant acinetobacter baumannii sequence type 191. PLoS One. 2016;11(9):e0162576. doi:10.1371/journal.pone.0162576 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

