Genetic variation in metronidazole metabolism and oxidative stress pathways in clinical Giardia lamblia assemblage A and B isolates
- PMID: 31190910
- PMCID: PMC6519707
- DOI: 10.2147/IDR.S177997
Genetic variation in metronidazole metabolism and oxidative stress pathways in clinical Giardia lamblia assemblage A and B isolates
Erratum in
-
Erratum: Genetic Variation in Metronidazole Metabolism and Oxidative Stress Pathways in Clinical Giardia lamblia Assemblage A and B Isolates [Corrigendum].Infect Drug Resist. 2021 Sep 14;14:3727-3728. doi: 10.2147/IDR.S338695. eCollection 2021. Infect Drug Resist. 2021. PMID: 34548796 Free PMC article.
Abstract
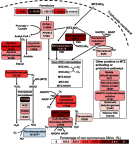
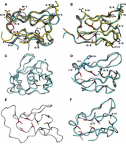
Purpose: Treatment-refractory Giardia cases have increased rapidly within the last decade. No markers of resistance nor a standardized susceptibility test have been established yet, but several enzymes and their pathways have been associated with metronidazole (MTZ) resistant Giardia. Very limited data are available regarding genetic variation in these pathways. We aimed to investigate genetic variation in metabolic pathway genes proposed to be involved in MTZ resistance in recently acquired, cultured clinical isolates. Methods: Whole genome sequencing of 12 assemblage A2 and 8 assemblage B isolates was done, to decipher genomic variation in Giardia. Twenty-nine genes were identified in a literature search and investigated for their single nucleotide variants (SNVs) in the coding/non-coding regions of the genes, either as amino acid changing (non-synonymous SNVs) or non-changing SNVs (synonymous). Results: In Giardia assemblage B, several genes involved in MTZ activation or oxidative stress management were found to have higher numbers of non-synonymous SNVs (thioredoxin peroxidase, nitroreductase 1, ferredoxin 2, NADH oxidase, nitroreductase 2, alcohol dehydrogenase, ferredoxin 4 and ferredoxin 1) than the average variation. For Giardia assemblage A2, the highest genetic variability was found in the ferredoxin 2, ferredoxin 6 and in nicotinamide adenine dinucleotide phosphate (NADPH) oxidoreductase putative genes. SNVs found in the ferredoxins and nitroreductases were analyzed further by alignment and homology modeling. SNVs close to the iron-sulfur cluster binding sites in nitroreductase-1 and 2 and ferredoxin 2 and 4 could potentially affect protein function. Flavohemoprotein seems to be a variable-copy gene, due to higher, but variable coverage compared to other genes investigated. Conclusion: In clinical Giardia isolates, genetic variability is common in important genes in the MTZ metabolizing pathway and in the management of oxidative and nitrosative stress and includes high numbers of non-synonymous SNVs. Some of the identified amino acid changes could potentially affect the respective proteins important in the MTZ metabolism.
Keywords: drug metabolism; ferredoxin; genetic analysis; genetic diversity; metronidazole genes; resistance.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
LinkOut - more resources
Full Text Sources

