The efficacy and safety of ceftaroline in the treatment of acute bacterial infection in pediatric patients - a systemic review and meta-analysis of randomized controlled trials
- PMID: 31190919
- PMCID: PMC6526920
- DOI: 10.2147/IDR.S199978
The efficacy and safety of ceftaroline in the treatment of acute bacterial infection in pediatric patients - a systemic review and meta-analysis of randomized controlled trials
Abstract
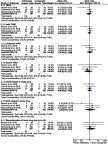
Objectives: This meta-analysis aims to assess the clinical efficacy and safety of ceftaroline in treating acute bacterial infections - community-acquired pneumonia (CAP) and skin and skin structure infection (SSSI) in pediatric patients. Methods: The Pubmed, Embase, ClinicalTrials.gov. and the Cochrane databases were searched up to December 31, 2018. Only randomized controlled trials (RCTs) evaluating ceftaroline and other comparators in the treatment of acute bacterial infection in pediatric patients were included. The primary outcome was the clinical cure rate and the secondary outcome was the risk of adverse event. Results: Three RCTs were included. Overall, ceftaroline had a clinical cure rate at end of therapy (EOT) and test of cure (TOC) similar to comparators in the treatment of acute bacterial infection (at EOT, OR, 1.93; 95% CI, 0.88-4.25, I2 =0%, and at TOC, OR, 1.36; 95% CI, 0.64-2.91, I2 =14%). In addition, ceftaroline had a clinical failure rate at EOT and TOC similar to comparators in the treatment of acute bacterial infection (at EOT, OR, 0.62; 95% CI, 0.22-1.76, I2 =0%, and at TOC, OR, 0.68; 95% CI, 0.24-1.91, I2 =0%). No significant differences were found for the risk of treatment-emergent adverse events (TEAE) in all and different degrees between ceftaroline and comparators (OR, 0.81; 95% CI, 0.37-1.78, I2 =56%). The risks of TEAE and severe adverse events related to study drug were similar between ceftaroline and comparators (TEAE related to study drug, OR, 0.98; 95% CI, 0.52-1.82, I2 =0%, severe adverse event related to study drug, OR, 1.09; 95% CI, 0.22-5.44, I2 =22%). Conclusions: The clinical efficacy of ceftaroline is as good as comparator therapy in the treatment of acute bacterial infections - CAP and SSSI, and this antibiotic is well tolerated as the comparators.
Keywords: acute bacterial infection; ceftaroline; community-acquired pneumonia; pediatric; skin and skin structure infection.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Efficacy and Safety of Ceftaroline for the Treatment of Community-Acquired Pneumonia: A Systemic Review and Meta-Analysis of Randomized Controlled Trials.J Clin Med. 2019 Jun 9;8(6):824. doi: 10.3390/jcm8060824. J Clin Med. 2019. PMID: 31181859 Free PMC article.
-
Ceftaroline Efficacy and Safety in Treatment of Complicated Skin and Soft Tissue Infection: A Systemic Review and Meta-Analysis of Randomized Controlled Trials.J Clin Med. 2019 May 31;8(6):776. doi: 10.3390/jcm8060776. J Clin Med. 2019. PMID: 31159264 Free PMC article.
-
Efficacy and Safety of Sitafloxacin in the Treatment of Acute Bacterial Infection: A Meta-analysis of Randomized Controlled Trials.Antibiotics (Basel). 2020 Mar 2;9(3):106. doi: 10.3390/antibiotics9030106. Antibiotics (Basel). 2020. PMID: 32131414 Free PMC article.
-
The Efficacy and Safety of Doripenem in the Treatment of Acute Bacterial Infections-A Systemic Review and Meta-Analysis of Randomized Controlled Trials.J Clin Med. 2019 Jul 2;8(7):958. doi: 10.3390/jcm8070958. J Clin Med. 2019. PMID: 31269697 Free PMC article. Review.
-
The efficacy and safety of omadacycline in treatment of acute bacterial infection: A systemic review and meta-analysis of randomized controlled trials.Medicine (Baltimore). 2019 Dec;98(51):e18426. doi: 10.1097/MD.0000000000018426. Medicine (Baltimore). 2019. PMID: 31861009 Free PMC article.
Cited by
-
Ceftaroline Fosamil for Treatment of Pediatric Complicated Skin and Soft Tissue Infections and Community-Acquired Pneumonia.Paediatr Drugs. 2021 Nov;23(6):549-563. doi: 10.1007/s40272-021-00468-w. Epub 2021 Aug 31. Paediatr Drugs. 2021. PMID: 34462863 Free PMC article. Review.
References
-
- Hedrick J. Acute bacterial skin infections in pediatric medicine: current issues in presentation and treatment. Paediatr Drugs. 2003;5 Suppl 1:35–46. - PubMed
-
- Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76. doi:10.1093/cid/cir531 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

