Comparative effectiveness of antifungal agents in patients with hematopoietic stem cell transplantation: a systematic review and network meta-analysis
- PMID: 31190920
- PMCID: PMC6526929
- DOI: 10.2147/IDR.S203579
Comparative effectiveness of antifungal agents in patients with hematopoietic stem cell transplantation: a systematic review and network meta-analysis
Abstract
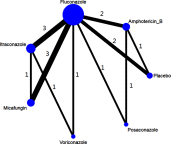
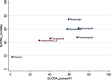
Purpose: The aim of this study was to use a network meta-analysis to evaluate the relative efficacy of various agents at preventing invasive fungal infections (IFIs). In this way, suitable prophylactic regimens may be selected for patients with hematopoietic stem cell transplantation (HSCT). Methods: We conducted a systematic review of randomized controlled trials comparing the prophylactic effects of two antifungal agents or an antifungal agent and a placebo administered to patients with HSCT. Relevant studies were found in the PubMed and Cochrane databases. Unpublished studies were collected from the ClinicalTrials.gov registry. Results: Sixteen two-arm studies were identified. Compared with placebo, all six antifungal agents (amphotericin B, fluconazole, itraconazole, micafungin, posaconazole, and voriconazole) presented with greater efficacy at controlling proven IFIs. OR ranged from 0.08 to 0.29. Voriconazole (surface under the cumulative ranking curve [SUCRA]=71.6%), posaconazole (SUCRA=68.9%), and itraconazole (SUCRA=64.7%) were the three top-ranking drugs for preventing proven IFIs. Itraconazole ranked highest (SUCRA=83.1%) and had the greatest efficacy at preventing invasive candidiasis. Posaconazole and micafungin were the two top-ranking drugs (SUCRA=81.3% and 78.4%, respectively) at preventing invasive aspergillosis. Micafungin and voriconazole were the drugs of choice because they lowered mortality more than the other agents (SUCRA=74.6% and 61.1%, respectively). Conclusion: This study is the first network meta-analysis to explore the prophylactic effects of antifungal agents in patients with HSCT. Voriconazole was the best choice for the prevention of proven IFIs in HSCT patients.
Keywords: antifungal agents; hematopoietic stem cell transplantation; network meta-analysis.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





Similar articles
-
Comparison of Antifungal Prophylaxis Drugs in Patients With Hematological Disease or Undergoing Hematopoietic Stem Cell Transplantation: A Systematic Review and Network Meta-analysis.JAMA Netw Open. 2020 Oct 1;3(10):e2017652. doi: 10.1001/jamanetworkopen.2020.17652. JAMA Netw Open. 2020. PMID: 33030550 Free PMC article.
-
Systemic antifungal prophylaxis after hematopoietic stem cell transplantation: a meta-analysis.Clin Ther. 2014 Feb 1;36(2):292-306.e1. doi: 10.1016/j.clinthera.2013.11.010. Epub 2014 Jan 17. Clin Ther. 2014. PMID: 24439393
-
Randomized trial of micafungin versus fluconazole as prophylaxis against invasive fungal infections in hematopoietic stem cell transplant recipients.J Infect. 2016 Nov;73(5):496-505. doi: 10.1016/j.jinf.2016.06.011. Epub 2016 Jul 7. J Infect. 2016. PMID: 27394404 Clinical Trial.
-
Newer antifungal agents micafungin and voriconazole for fungal infection prevention during hematopoietic cell transplantation: a meta-analysis.Eur Rev Med Pharmacol Sci. 2016;20(2):381-90. Eur Rev Med Pharmacol Sci. 2016. PMID: 26875911
-
Comparative Efficacy of Antifungal Agents Used in the Treatment of Oropharyngeal Candidiasis among HIV-Infected Adults: A Systematic Review and Network Meta-Analysis.J Fungi (Basel). 2021 Aug 5;7(8):637. doi: 10.3390/jof7080637. J Fungi (Basel). 2021. PMID: 34436176 Free PMC article. Review.
Cited by
-
The efficacy and safety of first-line monotherapies in primary therapy of invasive aspergillosis: a systematic review.Front Pharmacol. 2025 Jan 15;15:1530999. doi: 10.3389/fphar.2024.1530999. eCollection 2024. Front Pharmacol. 2025. PMID: 39881866 Free PMC article.
-
Efficacy and safety of posaconazole for the prevention of invasive fungal infections in immunocompromised patients: a systematic review with meta-analysis and trial sequential analysis.Sci Rep. 2020 Sep 3;10(1):14575. doi: 10.1038/s41598-020-71571-0. Sci Rep. 2020. PMID: 32884060 Free PMC article.
-
Clinical Usefulness of Susceptibility Breakpoints for Yeasts in the Treatment of Candidemia: A Noninterventional Study.J Fungi (Basel). 2020 Jun 2;6(2):76. doi: 10.3390/jof6020076. J Fungi (Basel). 2020. PMID: 32498436 Free PMC article.
References
-
- Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. doi:10.1086/651263 - DOI - PubMed
-
- Bow EJ, Vanness DJ, Slavin M, et al. Systematic review and mixed treatment comparison meta-analysis of randomized clinical trials of primary oral antifungal prophylaxis in allogeneic hematopoietic cell transplant recipients. BMC Infect Dis. 2015;15:128. doi:10.1186/s12879-015-0855-6 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

