Clinical utility of pembrolizumab in the management of advanced solid tumors: an evidence-based review on the emerging new data
- PMID: 31190995
- PMCID: PMC6527794
- DOI: 10.2147/CMAR.S151023
Clinical utility of pembrolizumab in the management of advanced solid tumors: an evidence-based review on the emerging new data
Abstract
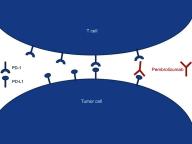
Pembrolizumab is a full-length human immunoglobulin G4 (IgG4) monoclonal antibody directed against the immune checkpoint PD-1 to remove its binding with PD-L1 and thus to restore an anti-tumor immune response of T cells. Pembrolizumab is one of the most advanced immune checkpoint inhibitors for cancer care. Apart from rare and serious adverse effects, its favorable tolerance profile enables to treat fragile patients who have often no other choice than best supportive care. The effective retained dose of pembrolizumab is a venous administration of 200 mg every 3 weeks until disease progression, intolerance or up to 24 months. Pembrolizumab has already proven its efficacy and thus obtained marketing authorization in so-called hot or hypermutated tumors or tumors expressing PD-L1 such as melanomas, non-small cell lung cancers, urothelial carcinomas, cervical cancer, etc. Pembrolizumab is also authorized in the United States in the treatment of mismatch repair-deficient tumors or with microsatellite instability. The current challenge is to expand its use in tumor types that are supposed to be less immunogenic, for example, by attempting to warm up the tumor microenvironment, or by combining pembrolizumab with other molecules. An acceptable toxicity profile of such combinations remains to explore. We review here the current indications of this drug, the main prognostic and predictive factors of its efficacy as well as the potential forthcoming indications.
Keywords: anti PD-1 antibody; immune checkpoint inhibitor; pembrolizumab.
Conflict of interest statement
Marie Robert received travel expenses. Marie Robert also reports honoraria from Merck and Novartis during the conduct of the study. Mario Campone acted in an advisory role for Novartis, Sanofi, Pierre Fabre, Lilly and Astra Zeneca outside the submitted work. The other authors report no conflicts of interest in this work.
Figures
References
-
- Sharp M, Corp D, Kenilworth NJ: KEYTRUDA® (pembrolizumab) Prescribing Information.pdf. Available from: http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed October 6, 2018.
-
- Ahamadi M, Freshwater T, Prohn M, et al. model-based characterization of the pharmacokinetics of pembrolizumab: a humanized anti-pd-1 monoclonal antibody in advanced solid tumors: pharmacokinetics of pembro in solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):49–57. doi:10.1002/psp4.12139 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials