Transverse myelitis associated with yellow fever vaccination
- PMID: 31191156
- PMCID: PMC6541067
- DOI: 10.1080/08998280.2019.1573405
Transverse myelitis associated with yellow fever vaccination
Abstract
Yellow fever is a mosquito-borne viral hemorrhagic fever endemic to sub-Saharan Africa and South America associated with significant morbidity and mortality. The use of a live attenuated vaccine can prevent yellow fever, but vaccine-associated neurologic disease has been reported and is a safety concern. We present the case of a previously healthy 35-year-old active-duty man who received the yellow fever vaccine prior to deployment and subsequently developed progressive neurologic dysfunction consistent with transverse myelitis.
Keywords: Transverse myelitis; yellow fever; yellow fever vaccine–associated neurologic disease.
Figures
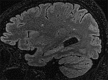
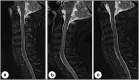
Similar articles
-
[YEL-AND meningoencephalitis in a 4-year-old boy consecutive to a yellow-fever vaccine].Arch Pediatr. 2014 Apr;21(4):384-7. doi: 10.1016/j.arcped.2014.01.014. Epub 2014 Mar 12. Arch Pediatr. 2014. PMID: 24630625 French.
-
[Yellow fever is still a current threat ?].Rev Prat. 2020 Mar;70(3):312-316. Rev Prat. 2020. PMID: 32877067 French.
-
Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2010 Jul 30;59(RR-7):1-27. MMWR Recomm Rep. 2010. PMID: 20671663
-
Yellow fever vaccine.Expert Rev Vaccines. 2005 Aug;4(4):553-74. doi: 10.1586/14760584.4.4.553. Expert Rev Vaccines. 2005. PMID: 16117712 Review.
-
[Yellow fever].Przegl Epidemiol. 2004;58 Suppl 1:101-5. Przegl Epidemiol. 2004. PMID: 15807166 Review. Polish.
Cited by
-
Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature.J Neurol. 2022 Mar;269(3):1121-1132. doi: 10.1007/s00415-021-10785-2. Epub 2021 Sep 5. J Neurol. 2022. PMID: 34482455 Free PMC article. Review.
-
Disease Resurgence, Production Capability Issues and Safety Concerns in the Context of an Aging Population: Is There a Need for a New Yellow Fever Vaccine?Vaccines (Basel). 2019 Nov 8;7(4):179. doi: 10.3390/vaccines7040179. Vaccines (Basel). 2019. PMID: 31717289 Free PMC article. Review.
References
-
- World Health Organization District Guidelines for Yellow Fever Surveillance (Publication no. WHO/EPI/GEN 98.09). Geneva, Switzerland: World Health Organization; 1998. Available at http://www.who.int/emc-documents/yellow_fever/whoepigen9809c.html.
-
- Monath TP, Barrett AD. Pathogenesis and pathophysiology of yellow fever. Adv Virus Res. 2003;60:343–395. - PubMed
-
- Cetron MS, Marfin AA, Julian KG, et al. . Yellow fever vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR Recomm Rep. 2002;51(RR-17):1–11. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
