Time-Course Evaluation of Iminodipropionitrile-Induced Liver and Kidney Toxicities in Rats: A Biochemical, Molecular and Histopathological Study
- PMID: 31191186
- PMCID: PMC6537673
- DOI: 10.1177/1559325819852233
Time-Course Evaluation of Iminodipropionitrile-Induced Liver and Kidney Toxicities in Rats: A Biochemical, Molecular and Histopathological Study
Abstract
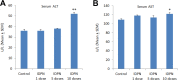
Iminodipropionitrile (IDPN) is known to produce axonopathy and vestibular hair cell degeneration. Recent histopathological studies have shown IDPN-induced liver and kidney toxicities in rodents; however, the associated mechanisms are not clearly understood. We investigated the role of proinflammatory cytokines in IDPN-induced liver and kidney toxicities in rats. Rats were treated with saline (control) and IDPN (100 mg/kg, intraperitoneally) daily for 1, 5, and 10 days, respectively. Animals were killed 24 hours after the last dose and liver and kidneys were collected for histopathology and interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α messenger RNA expression analysis. Serum aspartate aminotransferase and alanine aminotransferase activities were significantly increased after 10 doses of IDPN. The level of serum creatinine was initially increased after the first dose of IDPN but subsided on days 5 and 10. Blood urea nitrogen levels were significantly increased on days 5 and 10 following IDPN exposure. Histopathology showed dose-dependent hepatotoxicity in IDPN-treated rats. Iminodipropionitrile-induced expression of proinflammatory cytokines peaked after day 1 in liver and after day 5 in kidneys. In conclusion, repeated exposure of IDPN for 10 days produced significant structural and functional damages in rat liver whereas kidneys showed gradual recovery with time. These findings point toward the role of inflammatory mediators in IDPN-induced toxicity in rats.
Keywords: iminodipropionitrile; kidney; liver; proinflammatory cytokines; toxicity.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





Similar articles
-
Histological insights in iminodipropionitrile-induced toxicity in rats.Exp Toxicol Pathol. 2014 Mar;66(2-3):89-96. doi: 10.1016/j.etp.2013.11.004. Epub 2013 Dec 12. Exp Toxicol Pathol. 2014. PMID: 24332269
-
Pattern of neurobehavioral and organ-specific toxicities of β, β'-iminodipropionitrile in mice.Arch Med Sci. 2015 Oct 12;11(5):1137-44. doi: 10.5114/aoms.2015.54871. Arch Med Sci. 2015. PMID: 26528360 Free PMC article.
-
Exacerbation of iminodipropionitrile-induced behavioral toxicity, oxidative stress, and vestibular hair cell degeneration by gentamicin in rats.Neurotoxicol Teratol. 2000 Mar-Apr;22(2):213-20. doi: 10.1016/s0892-0362(99)00075-6. Neurotoxicol Teratol. 2000. PMID: 10758350
-
Neurovestibular toxicities of acrylonitrile and iminodipropionitrile in rats: a comparative evaluation of putative mechanisms and target sites.Toxicol Sci. 2009 May;109(1):124-31. doi: 10.1093/toxsci/kfp043. Epub 2009 Feb 25. Toxicol Sci. 2009. PMID: 19244277
-
Cysteamine attenuates iminodipropionitrile (IDPN) induced dyskinesia in rats.Int J Neurosci. 1995 Dec;83(3-4):165-75. doi: 10.3109/00207459508986336. Int J Neurosci. 1995. PMID: 8869425 Review.
Cited by
-
Protective effect of lyophilized sapodilla (Manilkara zapota) fruit extract against CCl4-induced liver damage in rats.Saudi J Biol Sci. 2020 Sep;27(9):2373-2379. doi: 10.1016/j.sjbs.2020.05.010. Epub 2020 May 11. Saudi J Biol Sci. 2020. PMID: 32884419 Free PMC article.
References
-
- Delay P, Pichot P, Thuillier J, Marquiset JP. Action de l’aminodipropionitrilesur le comportementmoteur de la souris blanche. CR Soc Biol. 1952;146:533–534. - PubMed
-
- McKay GF, Lalich JJ, Schilling ED, Strong FM. A crystalline “lathyrus factor” from Lathyrus odoratus. Arch Biochem Biophys. 1954;52(2):313–322. - PubMed
-
- Gagnaire F, Marignac B, Bonnet P. Relative neurotoxicological properties of five unsaturated aliphatic nitriles in rats. J Appl Toxicol. 1998;18(1): 25–31. - PubMed
-
- Tanii H, Hayashi M, Hashimoto K. Behavioral syndrome induced by allylnitrile, crotononitrile or 2-pentenenitrile in rats. Neuropharmacology. 1991;90:887–892. - PubMed
-
- Boadas-Vaello P, Riera J, Llorens J. Behavioral and pathological effects in the rat define two groups of neurotoxic nitriles. Toxicol Sci. 2005;88(2):456–466. - PubMed
LinkOut - more resources
Full Text Sources

