Blood Donor Screening for Hepatitis E Virus in the European Union
- PMID: 31191195
- PMCID: PMC6514502
- DOI: 10.1159/000499121
Blood Donor Screening for Hepatitis E Virus in the European Union
Abstract
This review article summarises hepatitis E virus (HEV) blood donation screening strategies in effect in the European Union (EU). Since 2012, eight EU countries have implemented HEV screening. Local rates of seroprevalence, RNA incidence, and molecular epidemiology are variable and not usually directly comparable. We report a range of HEV-RNA reactivity rates from 1 in 744 donations (France) to 1 in 8,636 donations (Wales) with an overall EU rate of 1 in 3,109 donations (3.2 million donations screened). HEV genotypes 3c, 3e, and 3f are the most frequently reported subtypes. In these 8 countries, both universal (n = 5) and selective (n = 3) screening policies have been introduced utilising either individual donation (ID; n = 1) or mini-pool (MP; n = 7; MP-6, -16, -24, and -96) testing. We also describe the Irish experience of HEV screening utilising an ID-NAT-based donor screening algorithm which intercepts donations even from those with low-level viraemia; 21 of 56 donors (37.5%) had a viral load (VL) < 100 IU/mL. We performed a MP-24 experiment which may prove useful to colleagues in relation to donor screening and associated blood component transmissibility. Irish results indicate that 59% of donors with a HEV-VL < 450 IU/mL may have screened negative in a MP-24.
Keywords: European Union; Hepatitis E virus; Nucleic acid testing; Viral load.
Figures
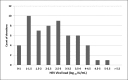
Similar articles
-
Prevalence of Acute Hepatitis E Virus Infections in Swiss Blood Donors 2018-2020.Viruses. 2024 May 8;16(5):744. doi: 10.3390/v16050744. Viruses. 2024. PMID: 38793625 Free PMC article.
-
Hepatitis E virus blood donor NAT screening: as much as possible or as much as needed?Transfusion. 2019 Feb;59(2):612-622. doi: 10.1111/trf.15058. Epub 2018 Dec 13. Transfusion. 2019. PMID: 30548866
-
Seven years (2015-2021) of blood donor screening for HEV-RNA in France: lessons and perspectives.Blood Transfus. 2023 Mar;21(2):110-118. doi: 10.2450/2022.0052-22. Epub 2022 Jul 25. Blood Transfus. 2023. PMID: 35969132 Free PMC article.
-
[Hepatitis E virus: Blood transfusion implications].Transfus Clin Biol. 2014 Nov;21(4-5):173-7. doi: 10.1016/j.tracli.2014.07.007. Epub 2014 Sep 27. Transfus Clin Biol. 2014. PMID: 25267201 Review. French.
-
Should HBV DNA NAT replace HBsAg and/or anti-HBc screening of blood donors?Transfus Clin Biol. 2004 Feb;11(1):26-32. doi: 10.1016/j.tracli.2003.12.003. Transfus Clin Biol. 2004. PMID: 14980546 Review.
Cited by
-
Transfusion-transmitted hepatitis E: What we know so far?World J Gastroenterol. 2022 Jan 7;28(1):47-75. doi: 10.3748/wjg.v28.i1.47. World J Gastroenterol. 2022. PMID: 35125819 Free PMC article. Review.
-
Hepatitis E Virus (HEV) Infection in the Context of the One Health Approach: A Systematic Review.Pathogens. 2025 Jul 16;14(7):704. doi: 10.3390/pathogens14070704. Pathogens. 2025. PMID: 40732750 Free PMC article. Review.
-
Undiagnosed liver diseases.Transl Gastroenterol Hepatol. 2021 Apr 5;6:28. doi: 10.21037/tgh.2020.04.04. eCollection 2021. Transl Gastroenterol Hepatol. 2021. PMID: 33824932 Free PMC article. Review.
-
Assessment of Hepatitis E virus transmission risks: a comprehensive review of cases among blood transfusion recipients and blood donors.Infect Ecol Epidemiol. 2024 Oct 15;14(1):2406834. doi: 10.1080/20008686.2024.2406834. eCollection 2024. Infect Ecol Epidemiol. 2024. PMID: 39421644 Free PMC article. Review.
-
Prevalence of Acute Hepatitis E Virus Infections in Swiss Blood Donors 2018-2020.Viruses. 2024 May 8;16(5):744. doi: 10.3390/v16050744. Viruses. 2024. PMID: 38793625 Free PMC article.
References
-
- Tedder RS, Ijaz S, Kitchen A, Ushiro-Lumb I, Tettmar KI, Hewitt P, et al. Hepatitis E risks: pigs or blood-that is the question. Transfusion. 2017 Feb;57((2)):267–72. - PubMed
-
- The European Directorate for the Quality of Medicines and Healthcare (EDQM) European Pharmacopoeia. 8th ed. Strasbourg: 32; 2016.
Publication types
LinkOut - more resources
Full Text Sources

