Safe-Testing Algorithm for Individual-Donation Nucleic Acid Testing: 10 Years of Experience in a Low-Prevalence Country
- PMID: 31191196
- PMCID: PMC6514477
- DOI: 10.1159/000499166
Safe-Testing Algorithm for Individual-Donation Nucleic Acid Testing: 10 Years of Experience in a Low-Prevalence Country
Abstract
Introduction: A highly sensitive and specific nucleic acid test (NAT) for the blood-borne viruses human immunodeficiency virus (HIV), hepatitis C (HCV), and hepatitis B (HBV) is essential for the safety of blood components. Since more than 2 decades, NAT screening of blood donations has become standard in developed countries that have implemented the individual-donation (ID-NAT) and mini-pool NAT (MP-NAT) approaches. With this powerful technique, confirmation of initial reactive (IR) NAT samples becomes a challenge. Different algorithms are currently in use to eliminate false reactive results. To show that the algorithm implemented in 2007, that uses repeat testing of IR samples in duplicate runs, is a safe strategy, especially in low endemic countries, data from a 10-year experience of ID-NAT were extensively analyzed when follow-up data were available.
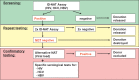
Methods: From July 2007 to December 2014, the Procleix Ultrio assay on a Procleix Tigris system, and from January 2015 to December 2017, the cobas MPX on a cobas 8800 platform, were used for ID-NAT screening. All IR samples were subjected to repeat testing in duplicate independent runs. Only when both tests remained negative were the products released. Donor data from the last 10 years were investigated retrospectively, looking for the reoccurrence of a reactive result in a follow-up sample. Only those donors with at least an x + 1 donation result were included for the confirmation of a false reactive result.
Results: From the 1,830,657 donations tested, 2,450 samples were IR (0.13%); only 228 were repeat reactive ([RR], 18 HIV, 61 HCV, and 149 HBV samples), and 2,222 were non-RR (0.12%). Follow-up data were available from 1,267 donors (57%) for further analysis. All except one of these donors were ID-NAT-negative in all follow-up samples. The one exception was from a donor who acquired a fresh HBV infection 10 years after the IR donation (in the x + 28 donation) and subsequently seroconverted. Subsequent serological tests from all succeeding donations (x + 1, x + 2, etc.) were negative in all the other cases, proving that no seroconversion took place after the IR ID-NAT result.
Conclusions: The algorithm to deal with IR ID-NAT donations using duplicate repeat testing is very safe and cost-effective in low-prevalence countries. There is no unnecessary destruction of blood products, no counseling of false reactive donors, and also no need to add further complexity to the screening algorithm.
Keywords: Blood-borne viruses; Individual donations; Mini-pools; Nucleic acid test.
Figures
Similar articles
-
Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa.Transfusion. 2009 Jun;49(6):1115-25. doi: 10.1111/j.1537-2995.2009.02110.x. Epub 2009 Feb 27. Transfusion. 2009. PMID: 19309474
-
A pilot study on screening blood donors with individual-donation nucleic acid testing in China.Blood Transfus. 2014 Apr;12(2):172-9. doi: 10.2450/2013.0095-13. Epub 2013 Oct 23. Blood Transfus. 2014. PMID: 24333061 Free PMC article. Clinical Trial.
-
Individual donor-nucleic acid testing for human immunodeficiency virus-1, hepatitis C virus and hepatitis B virus and its role in blood safety.Asian J Transfus Sci. 2015 Jul-Dec;9(2):199-202. doi: 10.4103/0973-6247.154250. Asian J Transfus Sci. 2015. PMID: 26420945 Free PMC article.
-
Should HBV DNA NAT replace HBsAg and/or anti-HBc screening of blood donors?Transfus Clin Biol. 2004 Feb;11(1):26-32. doi: 10.1016/j.tracli.2003.12.003. Transfus Clin Biol. 2004. PMID: 14980546 Review.
-
An international review of the characteristics of viral nucleic acid-amplification testing (NAT) reveals a trend towards the use of smaller pool sizes and individual donation NAT.Vox Sang. 2024 Jul;119(7):745-751. doi: 10.1111/vox.13617. Epub 2024 Mar 22. Vox Sang. 2024. PMID: 38516962 Review.
Cited by
-
Excluding Occult Hepatitis B Infection before Assigning False-Positive Status to Non-Repeatable NAT Reactivity: Concerning Stolz et al. "Safe-Testing Algorithm for Individual-Donation Nucleic Acid Testing: 10 Years of Experience in a Low-Prevalence Country" [Transfus Med Hemother. 2019 Apr;46(2):104-10].Transfus Med Hemother. 2020 Jun;47(3):272-274. doi: 10.1159/000502552. Epub 2019 Aug 29. Transfus Med Hemother. 2020. PMID: 32595432 Free PMC article. No abstract available.
-
Algorithms for the Testing of Tissue Donors for Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus.Transfus Med Hemother. 2021 Feb;48(1):12-22. doi: 10.1159/000513179. Epub 2020 Dec 22. Transfus Med Hemother. 2021. PMID: 33708048 Free PMC article.
-
High-Frequency Notable HBV Mutations Identified in Blood Donors With Occult Hepatitis B Infection From Heyuan City of Southern China.Front Immunol. 2022 May 13;13:754383. doi: 10.3389/fimmu.2022.754383. eCollection 2022. Front Immunol. 2022. PMID: 35634299 Free PMC article.
-
Prevalence of Occult Hepatitis B Virus Infection in Blood Donors with Negative ID-NAT in Switzerland.Transfus Med Hemother. 2022 Jul 6;49(6):338-345. doi: 10.1159/000525480. eCollection 2022 Dec. Transfus Med Hemother. 2022. PMID: 36654973 Free PMC article.
-
Infectious Screening of Blood Components: Is There Still a Need for Further Inventions?Transfus Med Hemother. 2019 Apr;46(2):65-66. doi: 10.1159/000499351. Epub 2019 Mar 14. Transfus Med Hemother. 2019. PMID: 31191191 Free PMC article. No abstract available.
References
-
- Bruhn R, Lelie N, Custer B, Busch M, Kleinman S, International NAT Study Group Prevalence of human immunodeficiency virus RNA and antibody in first-time, lapsed, and repeat blood donations across five international regions and relative efficacy of alternative screening scenarios. Transfusion. 2013 Oct;53((10 Pt 2)):2399–412. - PubMed
-
- Bruhn R, Lelie N, Busch M, Kleinman S, International NAT Study Group Relative efficacy of nucleic acid amplification testing and serologic screening in preventing hepatitis C virus transmission risk in seven international regions. Transfusion. 2015 Jun;55((6)):1195–205. - PubMed
-
- Stolz M, Tinguely C, Fontana S, Niederhauser C. Hepatitis B virus DNA viral load determination in hepatitis B surface antigen-negative Swiss blood donors. Transfusion. 2014 Nov;54((11)):2961–7. - PubMed
-
- Lelie N, Bruhn R, Busch M, Vermeulen M, Tsoi WC, Kleinman S, International NAT Study Group Detection of different categories of hepatitis B virus (HBV) infection in a multi-regional study comparing the clinical sensitivity of hepatitis B surface antigen and HBV-DNA testing. Transfusion. 2017 Jan;57((1)):24–35. - PubMed
-
- Vermeulen M, Lelie N. The current status of nucleic acid amplification technology in transfusion-transmitted infectious disease testing. ISBT Sci Ser. 2016;11(S2):123–8.
LinkOut - more resources
Full Text Sources