Impact of Sleep-Wake-Associated Neuromodulators and Repetitive Low-Frequency Stimulation on Human iPSC-Derived Neurons
- PMID: 31191238
- PMCID: PMC6549533
- DOI: 10.3389/fnins.2019.00554
Impact of Sleep-Wake-Associated Neuromodulators and Repetitive Low-Frequency Stimulation on Human iPSC-Derived Neurons
Abstract
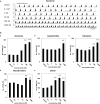
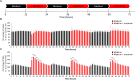
The cross-regional neurons in the brainstem, hypothalamus, and thalamus regulate the central nervous system, including the cerebral cortex, in a sleep-wake cycle-dependent manner. A characteristic brain wave, called slow wave, of about 1 Hz is observed during non-REM sleep, and the sleep homeostasis hypothesis proposes that the synaptic connection of a neural network is weakened during sleep. In the present study, in vitro human induced pluripotent stem cell (iPSC)-derived neurons, we investigated the responses to the neuromodulator known to be involved in sleep-wake regulation. We also determined whether long-term depression (LTD)-like phenomena could be induced by 1 Hz low-frequency stimulation (LFS), which is within the range of the non-REM sleep slow wave. A dose-dependent increase was observed in the number of synchronized burst firings (SBFs) when 0.1-1000 nM of serotonin, acetylcholine, histamine, orexin, or noradrenaline, all with increased extracellular levels during wakefulness, was administered to hiPSC-derived dopaminergic (DA) neurons. The number of SBFs repeatedly increased up to 5 h after 100 nM serotonin administration, inducing a 24-h rhythm cycle. Next, in human iPSC-derived glutamate neurons, 1 Hz LFS was administered four times for 15 min every 90 min. A significant reduction in both the number of firings and SBFs was observed in the 15 min immediately after LFS. Decreased frequency of spontaneous activity and recovery over time were repeatedly observed. Furthermore, we found that LFS attenuates synaptic connections, and particularly attenuates the strong connections in the neuronal network, and does not cause uniform attenuation. These results suggest sleep-wake states can be mimicked by cyclic neuromodulator administration and show that LTD-like phenomena can be induced by LFS in vitro human iPSC-derived neurons. These results could be applied in studies on the mechanism of slow waves during sleep or in an in vitro drug efficacy evaluation depending on sleep-wake state.
Keywords: circadian rhythms; human iPSC-derived neuron; long-term depression; low-frequency stimulation; micro-electrode array; neurotransmitters; non-REM sleep; wake.
Figures




Similar articles
-
[Selective stimulations and lesions of the rat brain nuclei as the models for research of the human sleep pathology mechanisms].Glas Srp Akad Nauka Med. 2011;(51):85-97. Glas Srp Akad Nauka Med. 2011. PMID: 22165729 Review. Serbian.
-
Induction of long-term potentiation and depression phenomena in human induced pluripotent stem cell-derived cortical neurons.Biochem Biophys Res Commun. 2016 Jan 22;469(4):856-62. doi: 10.1016/j.bbrc.2015.12.087. Epub 2015 Dec 21. Biochem Biophys Res Commun. 2016. PMID: 26718408
-
[Neurochemical mechanisms of sleep regulation].Glas Srp Akad Nauka Med. 2009;(50):97-109. Glas Srp Akad Nauka Med. 2009. PMID: 20666118 Review. Serbian.
-
[Major neurotransmitters involved in the regulation of sleep-wake cycle].Rev Invest Clin. 2012 Mar-Apr;64(2):182-91. Rev Invest Clin. 2012. PMID: 22991780 Review. Spanish.
-
Sleep Deprivation Distinctly Alters Glutamate Transporter 1 Apposition and Excitatory Transmission to Orexin and MCH Neurons.J Neurosci. 2018 Mar 7;38(10):2505-2518. doi: 10.1523/JNEUROSCI.2179-17.2018. Epub 2018 Feb 5. J Neurosci. 2018. PMID: 29431649 Free PMC article.
Cited by
-
Advances in construction and modeling of functional neural circuits in vitro.Neurochem Res. 2022 Sep;47(9):2529-2544. doi: 10.1007/s11064-022-03682-1. Epub 2022 Aug 9. Neurochem Res. 2022. PMID: 35943626 Free PMC article.
-
Multielectrode array characterization of human induced pluripotent stem cell derived neurons in co-culture with primary human astrocytes.bioRxiv [Preprint]. 2024 Mar 8:2024.03.04.583341. doi: 10.1101/2024.03.04.583341. bioRxiv. 2024. Update in: PLoS One. 2024 Jun 25;19(6):e0303901. doi: 10.1371/journal.pone.0303901. PMID: 38496655 Free PMC article. Updated. Preprint.
-
Multielectrode array characterization of human induced pluripotent stem cell derived neurons in co-culture with primary human astrocytes.PLoS One. 2024 Jun 25;19(6):e0303901. doi: 10.1371/journal.pone.0303901. eCollection 2024. PLoS One. 2024. PMID: 38917115 Free PMC article.
-
Astrocytes-The Ultimate Effectors of Long-Range Neuromodulatory Networks?Front Cell Neurosci. 2020 Sep 29;14:581075. doi: 10.3389/fncel.2020.581075. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192327 Free PMC article.
-
Human stem cell-based models to study synaptic dysfunction and cognition in schizophrenia: A narrative review.Schizophr Res. 2024 Nov;273:78-97. doi: 10.1016/j.schres.2023.02.029. Epub 2023 Mar 14. Schizophr Res. 2024. PMID: 36925354 Review.
References
-
- Charles B., Nemeroff (2004). The biochemical basis of neuropharmacology. Am. J. Psychiatry 161 379–379.
LinkOut - more resources
Full Text Sources

