Growth Inhibitory Effects of Dipotassium Glycyrrhizinate in Glioblastoma Cell Lines by Targeting MicroRNAs Through the NF-κB Signaling Pathway
- PMID: 31191251
- PMCID: PMC6546822
- DOI: 10.3389/fncel.2019.00216
Growth Inhibitory Effects of Dipotassium Glycyrrhizinate in Glioblastoma Cell Lines by Targeting MicroRNAs Through the NF-κB Signaling Pathway
Abstract
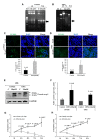
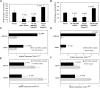
It has been shown that nuclear factor kappa-B (NF-κB) is constitutively activated in glioblastoma (GBM), suggesting that the pathway could be a therapeutic target. Glycyrrhetic acid (GA), a compound isolated from licorice (Glycyrrhiza glabra), has been shown to decrease cell viability and increases apoptosis in human cancer cell lines by NF-κB signaling pathway suppression. Dipotassium glycyrrhizinate (DPG), a dipotassium salt of GA, has anti-inflammatory properties without toxicity. The current study examined the effectiveness of DPG as an anti-tumor in U87MG and T98G GBM cell lines. Additionally, we assessed DPG as a candidate for combinational therapy in GBM with temozolomide (TMZ). Our results demonstrated that the viability of U87MG and T98G cells significantly decreased in a time- and dose-dependent manner after DPG treatment, and the apoptotic ratio of DPG-treated groups was significantly higher than that of control groups. In addition, DPG in combination with TMZ revealed synergistic effects. Furthermore, the expression of NF-κB-luciferase-reporter in transfected GBM cell lines was remarkably reduced after DPG exposure by up-regulating miR16 and miR146a, which down-regulate its target genes, IRAK2 and TRAF6. A reduced neuro-sphere formation was also observed after DPG in both GBM cells. In conclusion, DPG presented anti-tumoral effect on GBM cell lines through a decrease on proliferation and an increase on apoptosis. In addition, our data also suggest that DPG anti-tumoral effect is related to NF-κB suppression, where IRAK2- and TRAF6-mediating miR16 and miR146a, respectively, might be a potential therapeutic target of DPG.
Keywords: dipotassium glycyrrhizinate; glioblastoma; miR146a; miR16; nuclear factor kappa-B.
Figures




Similar articles
-
Dipotassium Glycyrrhizinate on Melanoma Cell Line: Inhibition of Cerebral Metastases Formation by Targeting NF-kB Genes-Mediating MicroRNA-4443 and MicroRNA-3620-Dipotassium Glycyrrhizinate Effect on Melanoma.Int J Mol Sci. 2022 Jun 29;23(13):7251. doi: 10.3390/ijms23137251. Int J Mol Sci. 2022. PMID: 35806253 Free PMC article.
-
Anti-Migratory Effect of Dipotassium Glycyrrhizinate on Glioblastoma Cell Lines: Microarray Data for the Identification of Key MicroRNA Signatures.Front Oncol. 2022 Aug 3;12:819599. doi: 10.3389/fonc.2022.819599. eCollection 2022. Front Oncol. 2022. PMID: 35992881 Free PMC article.
-
Natural Plant Compounds: Does Caffeine, Dipotassium Glycyrrhizinate, Curcumin, and Euphol Play Roles as Antitumoral Compounds in Glioblastoma Cell Lines?Front Neurol. 2022 Feb 17;12:784330. doi: 10.3389/fneur.2021.784330. eCollection 2021. Front Neurol. 2022. PMID: 35300350 Free PMC article. Review.
-
Nuclear factor I A promotes temozolomide resistance in glioblastoma via activation of nuclear factor κB pathway.Life Sci. 2019 Nov 1;236:116917. doi: 10.1016/j.lfs.2019.116917. Epub 2019 Oct 12. Life Sci. 2019. PMID: 31614149
-
Temozolomide resistance in glioblastoma multiforme.Genes Dis. 2016 May 11;3(3):198-210. doi: 10.1016/j.gendis.2016.04.007. eCollection 2016 Sep. Genes Dis. 2016. PMID: 30258889 Free PMC article. Review.
Cited by
-
Dipotassium Glycyrrhizinate on Melanoma Cell Line: Inhibition of Cerebral Metastases Formation by Targeting NF-kB Genes-Mediating MicroRNA-4443 and MicroRNA-3620-Dipotassium Glycyrrhizinate Effect on Melanoma.Int J Mol Sci. 2022 Jun 29;23(13):7251. doi: 10.3390/ijms23137251. Int J Mol Sci. 2022. PMID: 35806253 Free PMC article.
-
Xuanfei Baidu decoction in the treatment of coronavirus disease 2019 (COVID-19): Efficacy and potential mechanisms.Heliyon. 2023 Aug 19;9(9):e19163. doi: 10.1016/j.heliyon.2023.e19163. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809901 Free PMC article. Review.
-
MicroRNAs miR-16 and miR-519 control meningioma cell proliferation via overlapping transcriptomic programs shared with the RNA-binding protein HuR.Front Oncol. 2023 Aug 2;13:1158773. doi: 10.3389/fonc.2023.1158773. eCollection 2023. Front Oncol. 2023. PMID: 37601663 Free PMC article.
-
The Anti-Inflammatory Properties of Licorice (Glycyrrhiza glabra)-Derived Compounds in Intestinal Disorders.Int J Mol Sci. 2022 Apr 8;23(8):4121. doi: 10.3390/ijms23084121. Int J Mol Sci. 2022. PMID: 35456938 Free PMC article. Review.
-
Anti-Migratory Effect of Dipotassium Glycyrrhizinate on Glioblastoma Cell Lines: Microarray Data for the Identification of Key MicroRNA Signatures.Front Oncol. 2022 Aug 3;12:819599. doi: 10.3389/fonc.2022.819599. eCollection 2022. Front Oncol. 2022. PMID: 35992881 Free PMC article.
References
-
- Baker S. D., Wirth M., Statkevich P., Reidenberg P., Alton K., Sartorius S. E., et al. (1999). Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin. Cancer Res. 5 309–317. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

