Corticotectal Projections From the Premotor or Primary Motor Cortex After Cortical Lesion or Parkinsonian Symptoms in Adult Macaque Monkeys: A Pilot Tracing Study
- PMID: 31191260
- PMCID: PMC6540615
- DOI: 10.3389/fnana.2019.00050
Corticotectal Projections From the Premotor or Primary Motor Cortex After Cortical Lesion or Parkinsonian Symptoms in Adult Macaque Monkeys: A Pilot Tracing Study
Abstract
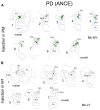
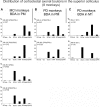
The corticotectal projections, together with the corticobulbar (corticoreticular) projections, work in parallel with the corticospinal tract (CST) to influence motoneurons in the spinal cord both directly and indirectly via the brainstem descending pathways. The tectospinal tract (TST) originates in the deep layers of the superior colliculus. In the present study, we analyzed the corticotectal projections from two motor cortical areas, namely the premotor cortex (PM) and the primary motor cortex (M1) in eight macaque monkeys subjected to either a cortical lesion of the hand area in M1 (n = 4) or Parkinson's disease-like symptoms PD (n = 4). A subgroup of monkeys with cortical lesion was subjected to anti-Nogo-A antibody treatment whereas all PD monkeys were transplanted with Autologous Neural Cell Ecosystems (ANCEs). The anterograde tracer BDA was used to label the axonal boutons both en passant and terminaux in the ipsilateral superior colliculus. Individual axonal boutons were charted in the different layers of the superior colliculus. In intact animals, we previously observed that corticotectal projections were denser when originating from PM than from M1. In the present M1 lesioned monkeys, as compared to intact ones the corticotectal projection originating from PM was decreased when treated with anti-Nogo-A antibody but not in untreated monkeys. In PD-like symptoms' monkeys, on the other hand, there was no consistent change affecting the corticotectal projection as compared to intact monkeys. The present pilot study overall suggests that the corticotectal projection is less affected by M1 lesion or PD symptoms than the corticoreticular projection previously reported in the same animals.
Keywords: Parkinson; anterograde tracing; brainstem; cortical lesion; motor cortex; non-human primate; spinal cord injury.
Figures


Similar articles
-
Loss of Motor Cortical Inputs to the Red Nucleus after CNS Disorders in Nonhuman Primates.J Neurosci. 2023 Mar 8;43(10):1682-1691. doi: 10.1523/JNEUROSCI.1942-22.2023. Epub 2023 Jan 24. J Neurosci. 2023. PMID: 36693756 Free PMC article.
-
Adaptation of the layer V supraspinal motor corticofugal projections from the primary (M1) and premotor (PM) cortices after CNS motor disorders in non-human primates: A survey.Transl Neurosci. 2024 Jun 7;15(1):20220342. doi: 10.1515/tnsci-2022-0342. eCollection 2024 Jan 1. Transl Neurosci. 2024. PMID: 38860225 Free PMC article. Review.
-
Ipsilateral corticotectal projections from the primary, premotor and supplementary motor cortical areas in adult macaque monkeys: a quantitative anterograde tracing study.Eur J Neurosci. 2017 Oct;46(8):2406-2415. doi: 10.1111/ejn.13709. Epub 2017 Oct 9. Eur J Neurosci. 2017. PMID: 28921678 Free PMC article.
-
Changes of motor corticobulbar projections following different lesion types affecting the central nervous system in adult macaque monkeys.Eur J Neurosci. 2018 Aug;48(4):2050-2070. doi: 10.1111/ejn.14074. Epub 2018 Aug 16. Eur J Neurosci. 2018. PMID: 30019432 Free PMC article.
-
Cortical and subcortical compensatory mechanisms after spinal cord injury in monkeys.Exp Neurol. 2012 May;235(1):152-61. doi: 10.1016/j.expneurol.2011.08.013. Epub 2011 Aug 23. Exp Neurol. 2012. PMID: 21884698 Review.
Cited by
-
Loss of Motor Cortical Inputs to the Red Nucleus after CNS Disorders in Nonhuman Primates.J Neurosci. 2023 Mar 8;43(10):1682-1691. doi: 10.1523/JNEUROSCI.1942-22.2023. Epub 2023 Jan 24. J Neurosci. 2023. PMID: 36693756 Free PMC article.
-
The role of eutherian-specific RTL1 in the nervous system and its implications for the Kagami-Ogata and Temple syndromes.Genes Cells. 2021 Mar;26(3):165-179. doi: 10.1111/gtc.12830. Epub 2021 Feb 16. Genes Cells. 2021. PMID: 33484574 Free PMC article.
-
The Cortical Motor System in the Domestic Pig: Origin and Termination of the Corticospinal Tract and Cortico-Brainstem Projections.Front Neuroanat. 2021 Nov 1;15:748050. doi: 10.3389/fnana.2021.748050. eCollection 2021. Front Neuroanat. 2021. PMID: 34790101 Free PMC article.
-
Adaptation of the layer V supraspinal motor corticofugal projections from the primary (M1) and premotor (PM) cortices after CNS motor disorders in non-human primates: A survey.Transl Neurosci. 2024 Jun 7;15(1):20220342. doi: 10.1515/tnsci-2022-0342. eCollection 2024 Jan 1. Transl Neurosci. 2024. PMID: 38860225 Free PMC article. Review.
-
The corticotegmental connectivity as an integral component of the descending extrapyramidal pathway: novel and direct structural evidence stemming from focused fiber dissections.Neurosurg Rev. 2021 Dec;44(6):3283-3296. doi: 10.1007/s10143-021-01489-2. Epub 2021 Feb 10. Neurosurg Rev. 2021. PMID: 33564983
References
-
- Badoud S., Borgognon S., Cottet J., Chatagny P., Moret V., Fregosi M., et al. . (2017). Effects of dorsolateral prefrontal cortex lesion on motor habit and performance assessed with manual grasping and control of force in macaque monkeys. Brain Struct. Funct. 222, 1193–1206. 10.1007/s00429-016-1268-z - DOI - PMC - PubMed
-
- Bloch J., Brunet J. F., McEntire C. R. S., Redmond D. E. (2014). Primate adult brain cell autotransplantation produces behavioral and biological recovery in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian St. Kitts monkeys. J. Comp. Neurol. 522, 2729–2740. 10.1002/cne.23579 - DOI - PubMed
-
- Borgognon S., Cottet J., Moret V., Chatagny P., Carrara L., Fregosi M., et al. (2019). Fine manual dexterity assessment after autologous neural cell ecosystems (ANCE) transplantation in a non-human primate model of Parkinson’s disease. Neurorehabil. Neural Repair - PubMed
-
- Borgognon S., Cottet J., Moret V., Chatagny P., Ginovart N., Antonescu C., et al. (2017). Enhancement of striatal dopaminergic function following autologous neural cell ecosystems (ANCE) transplantation in a non-human primate model of Parkinson’s disease. J. Alzheimers Dis. Parkinsonism 7:383 10.4172/2161-0460.1000383 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

