Mechanisms of PTPσ-Mediated Presynaptic Differentiation
- PMID: 31191292
- PMCID: PMC6540616
- DOI: 10.3389/fnsyn.2019.00017
Mechanisms of PTPσ-Mediated Presynaptic Differentiation
Abstract
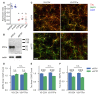
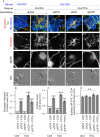
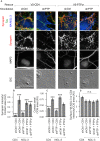
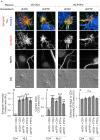
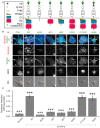
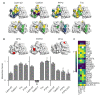
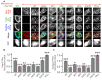
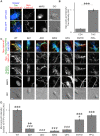
Formation of synapses between neurons depends in part on binding between axonal and dendritic cell surface synaptic organizing proteins, which recruit components of the developing presynaptic and postsynaptic specializations. One of these presynaptic organizing molecules is protein tyrosine phosphatase σ (PTPσ). Although the protein domains involved in adhesion between PTPσ and its postsynaptic binding partners are known, the mechanisms by which it signals into the presynaptic neuron to recruit synaptic vesicles and other necessary components for regulated transmitter release are not well understood. One attractive candidate to mediate this function is liprin-α, a scaffolding protein with well-established roles at the synapse. We systematically mutated residues of the PTPσ intracellular region (ICR) and used the yeast dihydrofolate reductase (DHFR) protein complementation assay to screen for disrupted interactions between these mutant forms of PTPσ and its various binding partners. Using a molecular replacement strategy, we show that disrupting the interaction between PTPσ and liprin-α, but not between PTPσ and itself or another binding partner, caskin, abolishes presynaptic differentiation. Furthermore, phosphatase activity of PTPσ and binding to extracellular heparan sulfate (HS) proteoglycans are dispensable for presynaptic induction. Previous reports have suggested that binding between PTPσ and liprin-α is mediated by the PTPσ membrane-distal phosphatase-like domain. However, we provide evidence here that both of the PTPσ phosphatase-like domains mediate binding to liprin-α and are required for PTPσ-mediated presynaptic differentiation. These findings further our understanding of the mechanistic basis by which PTPσ acts as a presynaptic organizer.
Keywords: LAR-RPTP; adhesion proteins; liprin; phosphatase; scaffolding proteins; synapse; synaptogenesis.
Figures
Similar articles
-
LAR-RPTPs Directly Interact with Neurexins to Coordinate Bidirectional Assembly of Molecular Machineries.J Neurosci. 2020 Oct 28;40(44):8438-8462. doi: 10.1523/JNEUROSCI.1091-20.2020. Epub 2020 Oct 9. J Neurosci. 2020. PMID: 33037075 Free PMC article.
-
PTPσ Drives Excitatory Presynaptic Assembly via Various Extracellular and Intracellular Mechanisms.J Neurosci. 2018 Jul 25;38(30):6700-6721. doi: 10.1523/JNEUROSCI.0672-18.2018. Epub 2018 Jun 22. J Neurosci. 2018. PMID: 29934346 Free PMC article.
-
Neurotrophin-3 Enhances the Synaptic Organizing Function of TrkC-Protein Tyrosine Phosphatase σ in Rat Hippocampal Neurons.J Neurosci. 2015 Sep 9;35(36):12425-31. doi: 10.1523/JNEUROSCI.1330-15.2015. J Neurosci. 2015. PMID: 26354911 Free PMC article.
-
Emerging roles of the neurotrophin receptor TrkC in synapse organization.Neurosci Res. 2017 Mar;116:10-17. doi: 10.1016/j.neures.2016.09.009. Epub 2016 Sep 30. Neurosci Res. 2017. PMID: 27697534 Review.
-
Type IIa RPTPs and Glycans: Roles in Axon Regeneration and Synaptogenesis.Int J Mol Sci. 2021 May 24;22(11):5524. doi: 10.3390/ijms22115524. Int J Mol Sci. 2021. PMID: 34073798 Free PMC article. Review.
Cited by
-
PTPσ Controls Presynaptic Organization of Neurotransmitter Release Machinery at Excitatory Synapses.iScience. 2020 Jun 26;23(6):101203. doi: 10.1016/j.isci.2020.101203. Epub 2020 May 28. iScience. 2020. PMID: 32516721 Free PMC article.
-
Presynaptic PTPσ regulates postsynaptic NMDA receptor function through direct adhesion-independent mechanisms.Elife. 2020 Mar 6;9:e54224. doi: 10.7554/eLife.54224. Elife. 2020. PMID: 32142410 Free PMC article.
-
Regulation of autophagy by inhibitory CSPG interactions with receptor PTPσ and its impact on plasticity and regeneration after spinal cord injury.Exp Neurol. 2020 Jun;328:113276. doi: 10.1016/j.expneurol.2020.113276. Epub 2020 Mar 4. Exp Neurol. 2020. PMID: 32145250 Free PMC article. Review.
-
Liprins in oncogenic signaling and cancer cell adhesion.Oncogene. 2021 Nov;40(46):6406-6416. doi: 10.1038/s41388-021-02048-1. Epub 2021 Oct 15. Oncogene. 2021. PMID: 34654889 Free PMC article. Review.
-
PTPσ Knockdown in Lampreys Impairs Reticulospinal Axon Regeneration and Neuronal Survival After Spinal Cord Injury.Front Cell Neurosci. 2020 Mar 19;14:61. doi: 10.3389/fncel.2020.00061. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32265663 Free PMC article.
References
-
- Ackley B. D., Harrington R. J., Hudson M. L., Williams L., Kenyon C. J., Chisholm A. D., et al. . (2005). The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J. Neurosci. 25, 7517–7528. 10.1523/JNEUROSCI.2010-05.2005 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

