Pharmacological Potential of Cilostazol for Alzheimer's Disease
- PMID: 31191308
- PMCID: PMC6540873
- DOI: 10.3389/fphar.2019.00559
Pharmacological Potential of Cilostazol for Alzheimer's Disease
Abstract
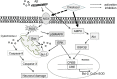
Alzheimer's disease (AD), a slow progressive form of dementia, is clinically characterized by cognitive dysfunction and memory impairment and neuropathologically characterized by the accumulation of extracellular plaques containing amyloid β-protein (Aβ) and neurofibrillary tangles containing tau in the brain, with neuronal degeneration and high level of oxidative stress. The current treatments for AD, e.g., acetylcholinesterase inhibitors (AChEIs), have efficacies limited to symptom improvement. Although there are various approaches to the disease modifying therapies of AD, none of them can be used alone for actual treatment, and combination therapy may be needed for amelioration of the progression. There are reports that cilostazol (CSZ) suppressed cognitive decline progression in patients with mild cognitive impairment or stable AD receiving AChEIs. Previously, we showed that CSZ suppressed Aβ-induced neurotoxicity in SH-SY5Y cells via coincident inhibition of oxidative stress, as demonstrated by reduced activity of nicotinamide adenine dinucleotide phosphate oxidase, accumulation of reactive oxygen species, and signaling of mitogen-activated protein kinase. CSZ also rescued cognitive impairment and promoted soluble Aβ clearance in a mouse model of cerebral amyloid angiopathy. Mature Aβ fibrils have long been considered the primary neurodegenerative factors in AD; however, recent evidence indicates soluble oligomers to initiate the neuronal and synaptic dysfunction related to AD and other protein-misfolding diseases. Further underscoring the potential of CSZ for AD treatment, we recently described the inhibitory effects of CSZ on Aβ oligomerization and aggregation in vitro. In this review, we discuss the possibility of CSZ as a potential disease-modifying therapy for the prevention or delay of AD.
Keywords: Alzheimer’s disease; amyloid β-protein; cilostazol; neurotoxicity; oligomer.
Figures

Similar articles
-
Cilostazol Suppresses Aβ-induced Neurotoxicity in SH-SY5Y Cells through Inhibition of Oxidative Stress and MAPK Signaling Pathway.Front Aging Neurosci. 2017 Oct 17;9:337. doi: 10.3389/fnagi.2017.00337. eCollection 2017. Front Aging Neurosci. 2017. PMID: 29089887 Free PMC article.
-
Supratherapeutic concentrations of cilostazol inhibits β-amyloid oligomerization in vitro.Neurosci Lett. 2018 Jun 11;677:19-25. doi: 10.1016/j.neulet.2018.04.032. Epub 2018 Apr 21. Neurosci Lett. 2018. PMID: 29684530
-
Advancing combination treatment with cilostazol and caffeine for Alzheimer's disease in high fat-high fructose-STZ induced model of amnesia.Eur J Pharmacol. 2022 Apr 15;921:174873. doi: 10.1016/j.ejphar.2022.174873. Epub 2022 Mar 10. Eur J Pharmacol. 2022. PMID: 35283111
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Therapeutic Strategies Targeting Amyloid-β in Alzheimer's Disease.Curr Alzheimer Res. 2019;16(5):418-452. doi: 10.2174/1567205016666190321163438. Curr Alzheimer Res. 2019. PMID: 30907320 Review.
Cited by
-
LigAdvisor: a versatile and user-friendly web-platform for drug design.Nucleic Acids Res. 2021 Jul 2;49(W1):W326-W335. doi: 10.1093/nar/gkab385. Nucleic Acids Res. 2021. PMID: 34023895 Free PMC article.
-
Inhibition of amyloid beta oligomer accumulation by NU-9: A unifying mechanism for the treatment of neurodegenerative diseases.Proc Natl Acad Sci U S A. 2025 Mar 11;122(10):e2402117122. doi: 10.1073/pnas.2402117122. Epub 2025 Mar 3. Proc Natl Acad Sci U S A. 2025. PMID: 40030015
-
Sex Differences in the Preventive Effect of Cardiovascular and Metabolic Therapeutics on Dementia.Biomol Ther (Seoul). 2023 Nov 1;31(6):583-598. doi: 10.4062/biomolther.2023.115. Biomol Ther (Seoul). 2023. PMID: 37899743 Free PMC article. Review.
-
Phosphodiesterase Inhibitors for Alzheimer's Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale.J Alzheimers Dis Rep. 2020 Jun 16;4(1):185-215. doi: 10.3233/ADR-200191. J Alzheimers Dis Rep. 2020. PMID: 32715279 Free PMC article. Review.
-
Cilostazol for Secondary Stroke Prevention: History, Evidence, Limitations, and Possibilities.Stroke. 2021 Oct;52(10):e635-e645. doi: 10.1161/STROKEAHA.121.035002. Epub 2021 Sep 14. Stroke. 2021. PMID: 34517768 Free PMC article. Review.
References
-
- Akiyama H., Kudo S., Shimizu T. (1985). The absorption, distribution and excretion of a new antithrombotic and vasodilating agent, cilostazol, in rat, rabbit, dog and man. Arzneimittelforschung 35, 1124–1132. PMID: - PubMed
Publication types
LinkOut - more resources
Full Text Sources

